Team:SJTU-BioX-Shanghai/Project/project2.2
From 2012.igem.org
AleAlejandro (Talk | contribs) |
|||
| (11 intermediate revisions not shown) | |||
| Line 24: | Line 24: | ||
<td valign="top" width="750"> | <td valign="top" width="750"> | ||
__NOTOC__ | __NOTOC__ | ||
| - | =Membrane Accelerator - Fatty Acid and Alkane Biosynthesis= | + | =Membrane Accelerator<br><br> - Fatty Acid and Alkane Biosynthesis= |
{{Template:12SJTU_part_summary_head}} | {{Template:12SJTU_part_summary_head}} | ||
*'''State of the art''' | *'''State of the art''' | ||
| Line 39: | Line 39: | ||
*'''Achievements''' | *'''Achievements''' | ||
| - | + | ''4 enzyme-bearing fusion proteins'' constructed that localize on membrane and assemble orderly. | |
| - | + | ''50% yield increase'' of fatty acids sinply through localizing single TesA onto ''E.coli'''s inner membrane. | |
| - | + | ''24 fold of yield increase'' of fatty acids by localizing TesA, FabG, FabZ, and FabI onto ''E.coli'''s inner membrane. | |
| - | + | ''fatty-acid-producing Membrane Accelerator upgrades'' by introducing additional two enzymes that convert fatty acids into alkane. | |
{{Template:12SJTU_part_summary_foot}} | {{Template:12SJTU_part_summary_foot}} | ||
| Line 105: | Line 105: | ||
===Membrane Accelerator in Alkane Biosynthesis=== | ===Membrane Accelerator in Alkane Biosynthesis=== | ||
| + | |||
| + | '''Flexibility of Reaction Space''' | ||
| + | |||
| + | [[File:12SJTU alkanereactionspace-1.jpg|350px|right|thumb|''Fig.7'' shows we can easily change the reaction space from cytoplasm to periplasm with ''Membrane Accelerator''.]] | ||
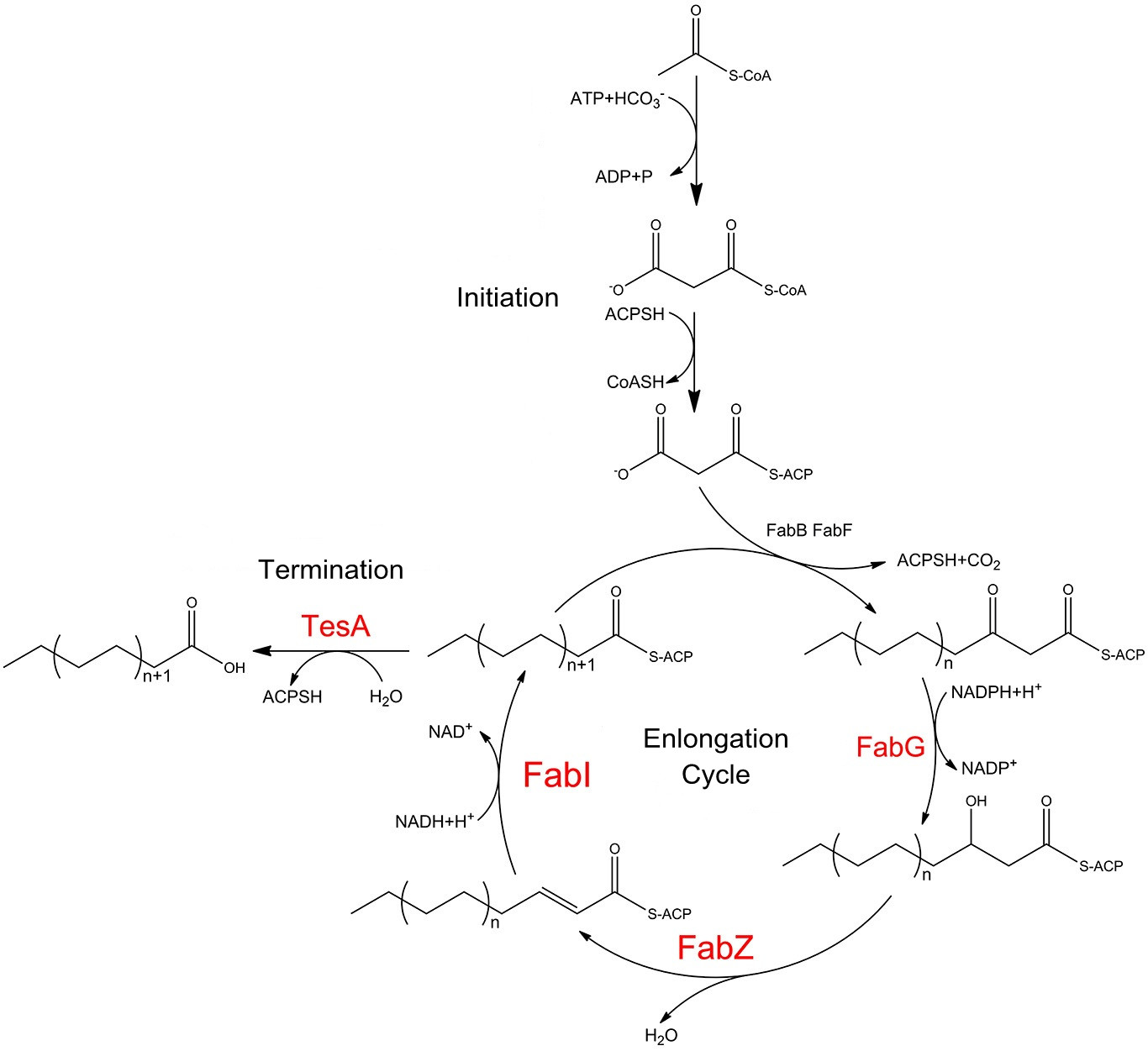
As mentioned above, fatty acyl-ACP is produced in ''E.coli'' by a nine-component enzyme system, in which FabG, FabZ, FabI play crucial part. AAR and ADC could then convert fatty acyl-ACP into alkane, the main constituents of diesel fuel. At first, we planned to link FabG, FabZ, FabI, AAR and ADC with constitutively assembling membrane anchors to create a new ''Membrane Accelerator''. In this case, all enzymes face cytoplasm. However, a problem would occur because alkane is very hard to be exported to periplasm. To much alkane would pose stress on ''E.coli''. | As mentioned above, fatty acyl-ACP is produced in ''E.coli'' by a nine-component enzyme system, in which FabG, FabZ, FabI play crucial part. AAR and ADC could then convert fatty acyl-ACP into alkane, the main constituents of diesel fuel. At first, we planned to link FabG, FabZ, FabI, AAR and ADC with constitutively assembling membrane anchors to create a new ''Membrane Accelerator''. In this case, all enzymes face cytoplasm. However, a problem would occur because alkane is very hard to be exported to periplasm. To much alkane would pose stress on ''E.coli''. | ||
| - | As we know, aldehyde is more easily to be exported through cell membrane than alkane. Considering the uniqueness and adaptability of Membrane Scaffold system, we decided to place ADC in the periplasmic function domain of Membrane Anchor, as shown in ''Fig.7''. Thus, after the production of fatty aldehyde by AAR in cytoplasm near inner membrane, the long chain aldehyde molecule is readily exported to periplasm. In periplasm, fatty aldehyde is processed by ADC to be converted into free alkane. The upgraded ''Membrane Accelerator'' is expected to facilitate alkane production in ''E.coli'' not only by aggregating enzymes but also by improving alkane exportation. | + | As we know, aldehyde is more easily to be exported through cell membrane than alkane. Considering the uniqueness and adaptability of Membrane Scaffold system, we decided to place ADC in the periplasmic function domain of Membrane Anchor, as shown in ''Fig.7''. Thus, after the production of fatty aldehyde by AAR in cytoplasm near inner membrane, the long chain aldehyde molecule is readily exported to periplasm. In periplasm, fatty aldehyde is processed by ADC to be converted into free alkane. The upgraded ''Membrane Accelerator'' is expected to facilitate alkane production in ''E.coli'' not only by aggregating enzymes but also by improving alkane exportation. |
| - | [[File:12SJTU Alkane accelerator.jpg|450px|center|thumb|''Fig. | + | We have constructed corresponding fusion proteins shown in ''Fig.8''. Full result and data analysis will be out soon. |
| + | |||
| + | [[File:12SJTU Alkane accelerator.jpg|450px|center|thumb|''Fig.8'' demonstrates the construction details of ''Membrane Accelerator'' for use of alkane synthesis.]] | ||
==Result and Discussion== | ==Result and Discussion== | ||
| - | === | + | ===Membrane Accelerator in Fatty Acid Biosynthesis=== |
| + | |||
| + | '''Overview''' | ||
| + | |||
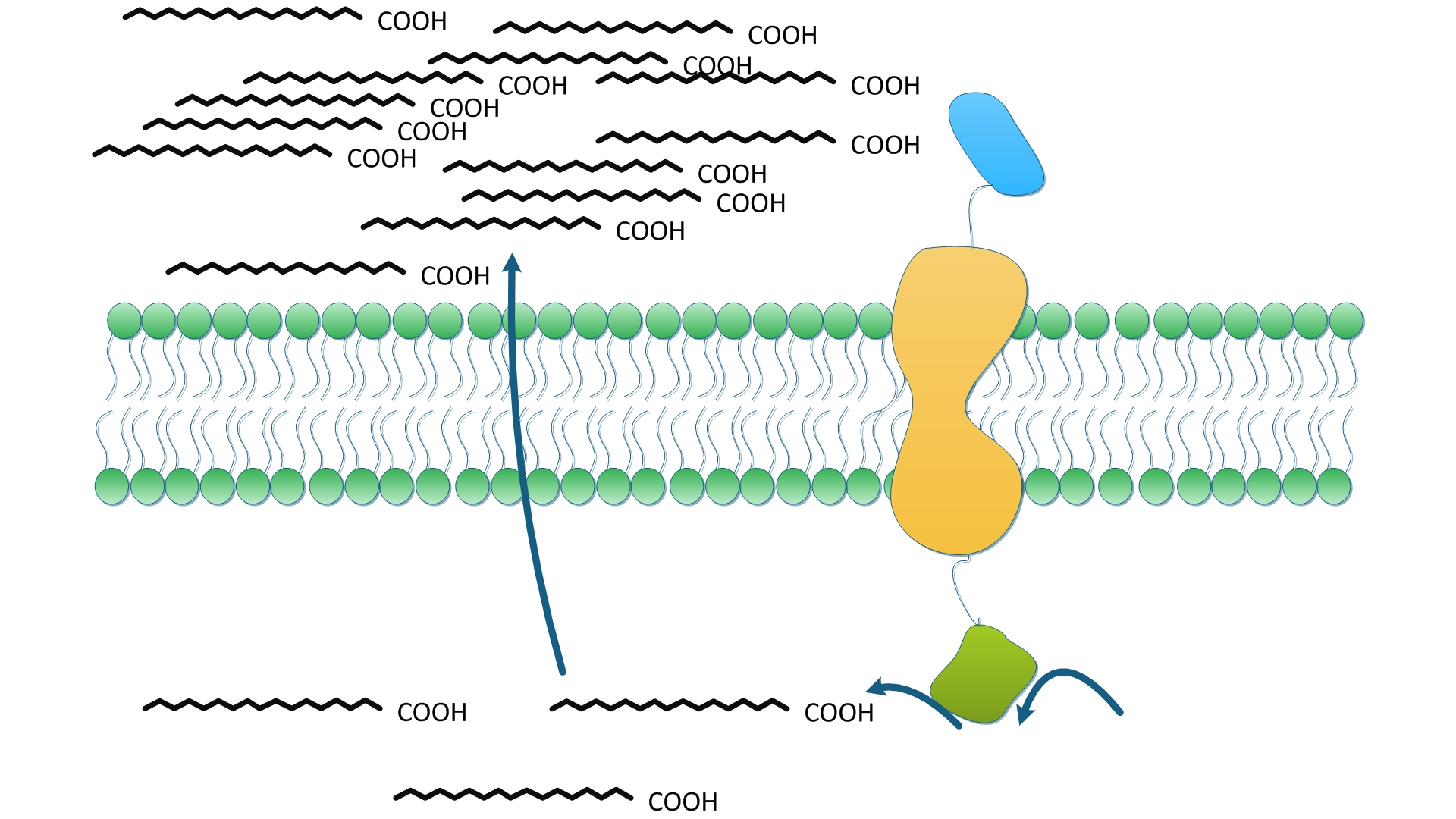
Excitingly, the fatty acid biosynthesis was accelerated sharply by recruiting ''membrane accelerator''. The introduction of TesA on membrane alone results in '''50% increase in fatty acids yield'''. By gathering FabG, FabI, FabZ and TesA on the membrane, the production of fatty acid was '''enhanced significantly by 9 fold '''compared with ''E.coli'' overexpressing the same amount of corresponding cytoplasmic enzymes. The statistical data strongly support the superiority of ''Membrane Accelerator''. | Excitingly, the fatty acid biosynthesis was accelerated sharply by recruiting ''membrane accelerator''. The introduction of TesA on membrane alone results in '''50% increase in fatty acids yield'''. By gathering FabG, FabI, FabZ and TesA on the membrane, the production of fatty acid was '''enhanced significantly by 9 fold '''compared with ''E.coli'' overexpressing the same amount of corresponding cytoplasmic enzymes. The statistical data strongly support the superiority of ''Membrane Accelerator''. | ||
| Line 126: | Line 135: | ||
Statistical data are shown as below and GC-MS data are attached to https://2012.igem.org/Team:SJTU-BioX-Shanghai/Parts | Statistical data are shown as below and GC-MS data are attached to https://2012.igem.org/Team:SJTU-BioX-Shanghai/Parts | ||
| - | |||
'''Assay of the Priority to Exportation''' | '''Assay of the Priority to Exportation''' | ||
| Line 132: | Line 140: | ||
The construction detail of experimental group is shown in ''Fig.4''. Control group is ''E.coli'' expressing the same amount of diffusing cytoplasmic TesA. | The construction detail of experimental group is shown in ''Fig.4''. Control group is ''E.coli'' expressing the same amount of diffusing cytoplasmic TesA. | ||
| - | TesA is responsible for the hydrolysis of fatty acyl-ACP, with special affinity to C16- and C18- precursor. As ''Fig. | + | TesA is responsible for the hydrolysis of fatty acyl-ACP, with special affinity to C16- and C18- precursor. As ''Fig.9 A'' indicates, 20 hours after induction, ''E.coli'' with membrane anchored TesA experienced a 50% increase in C-16, C-18 fatty acids content and total fatty acids (''Fig.9 B'') in supernatant, compared with ''E.coli'' with free TesA. |
| - | [[File:12SJTU-TesA-supernatant.png|thumb|center|750px|''Fig. | + | [[File:12SJTU-TesA-supernatant.png|thumb|center|750px|''Fig.9'' shows fatty acid content in supernatant of three groups to evaluate the advantages of membrane anchored TesA. A indicates C16/C18 fatty acids content among three groups. B stands for the total amount of fatty acids among three groups.]] |
Fatty acids yielded in supernatant went up to 0.71mg/(L·OD). The result supports that membrane anchored TesA could efficiently transfer fatty acyl-ACP into desirable fatty acids right beneath inner membrane, thus making it much easier for the final products to diffuse into periplasmic space. | Fatty acids yielded in supernatant went up to 0.71mg/(L·OD). The result supports that membrane anchored TesA could efficiently transfer fatty acyl-ACP into desirable fatty acids right beneath inner membrane, thus making it much easier for the final products to diffuse into periplasmic space. | ||
| - | [[File:12SJTU-TesA-sediment.png|thumb|center|750px|''Fig. | + | [[File:12SJTU-TesA-sediment.png|thumb|center|750px|''Fig.10'' shows fatty acid content in the sediment of three groups to evaluate the advantages of membrane anchored TesA. A indicates C16/C18 fatty acids content among three groups. B stands for the total amount of fatty acids among three groups.]] |
| - | ''Fig. | + | ''Fig.10'', on the other hand, showed fatty acids content in sedimentation also went up by 40%, up to 5.02mg/(L·OD). The result is still within our expectation since fatty acyl-ACP has been removed from the reaction system to form free fatty acids. As a result, the chemical equilibrium shifts and more fatty acids would be accumulated. |
We also witnessed a slight decrease in ''E.coli expressing'' free TesA compared with the wildtype, which testifies the statement in a previous study that high levels of TesA inhibits fatty acids synthesis activity but could enhance the activity at low concentrations. | We also witnessed a slight decrease in ''E.coli expressing'' free TesA compared with the wildtype, which testifies the statement in a previous study that high levels of TesA inhibits fatty acids synthesis activity but could enhance the activity at low concentrations. | ||
| Line 152: | Line 160: | ||
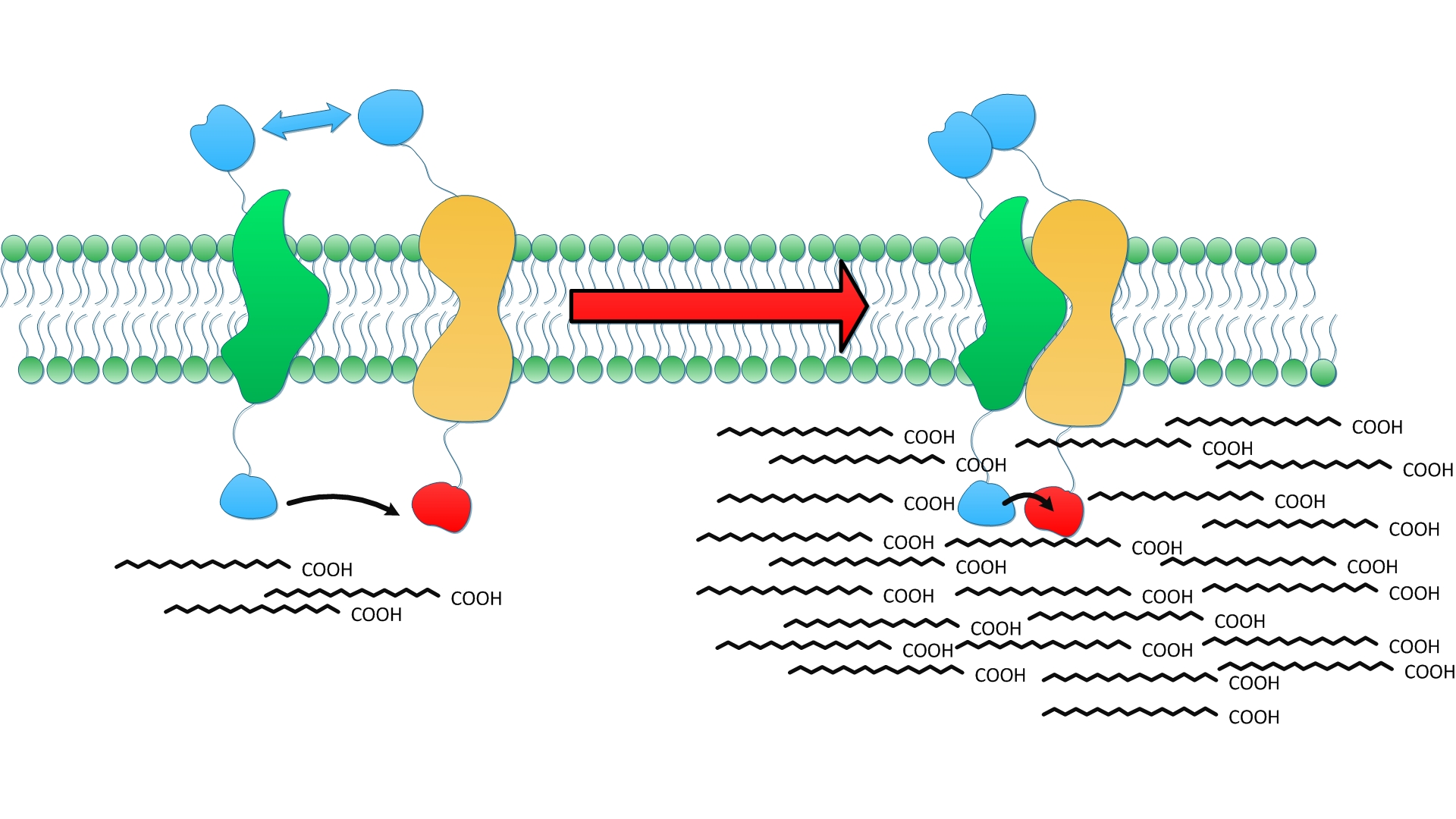
The construction detail of experimental group is shown in ''Fig.5''. Control group is ''E.coli'' expressing the same amount of diffusing cytoplasmic TesA, FabG, FabI and FabZ. | The construction detail of experimental group is shown in ''Fig.5''. Control group is ''E.coli'' expressing the same amount of diffusing cytoplasmic TesA, FabG, FabI and FabZ. | ||
| - | [[File:12SJTU-fattyAGZI.png|thumb|center|750px|''Fig. | + | [[File:12SJTU-fattyAGZI.png|thumb|center|750px|''Fig.11'' shows fatty acid content in supernatant of three groups to evaluate the advantages of membrane anchored TesA,FabG,FabZ and FabI. A indicates C16/C18 fatty acids content. B stands for the total amount of fatty acids. C shows the changes in products diversity.]] |
| - | Cluster of FabG, FabI and FabZ provides C16- and C18 specific TesA with sufficient amount of fatty acyl-ACP to hydrolyze and release. Therefore, we witnessed a tremendous growth in the turnover of fatty acids with C16 and C18 skeleton in the supernatant.(Fig. | + | Cluster of FabG, FabI and FabZ provides C16- and C18 specific TesA with sufficient amount of fatty acyl-ACP to hydrolyze and release. Therefore, we witnessed a tremendous growth in the turnover of fatty acids with C16 and C18 skeleton in the supernatant.(Fig.11 A) |
| - | Moreover, fatty acid with C14 skeleton was first detected in ''E.coli'' expressing membrane anchored enzymes compared with ones with free enzymes. It is probably because the high productivity of membrane anchored TesA quickly released free fatty acid. Monounsaturated fatty acids also emerge in considerable amount for the first time since TesA is located so closely to the cluster of FabG, FabI and FabZ that it catches intermediate with C16 and C18 skeleton even before they are reduced.(Fig. | + | Moreover, fatty acid with C14 skeleton was first detected in ''E.coli'' expressing membrane anchored enzymes compared with ones with free enzymes. It is probably because the high productivity of membrane anchored TesA quickly released free fatty acid. Monounsaturated fatty acids also emerge in considerable amount for the first time since TesA is located so closely to the cluster of FabG, FabI and FabZ that it catches intermediate with C16 and C18 skeleton even before they are reduced.(Fig.11 C) |
| - | Augment in both diversity and amount of fatty acids led to 24 fold increase in total fatty acid yield compared with wild type and 9 fold compared with ''E.coli'' expressing diffusing cytoplasmic enzymes. (Fig. | + | Augment in both diversity and amount of fatty acids led to 24 fold increase in total fatty acid yield compared with wild type and 9 fold compared with ''E.coli'' expressing diffusing cytoplasmic enzymes. (Fig.11 B) The exciting result convincingly proves that membrane scaffold enhances protein interaction and cluster of enzymes makes reaction faster to a surprising extent. Products accumulated near inner membrane can traval a shorter distance to be exported to membrane. |
| - | [[File:12SJTU-123.png|thumb|center|750px|''Fig. | + | [[File:12SJTU-123.png|thumb|center|750px|''Fig.12'' shows fatty acid content in sediment of three groups to evaluate the advantages of ''Membrane Accelerator''. A indicates C16/C18 fatty acids content. B stands for the total amount of fatty acids.]] |
The fatty acids yields increase in sediment is relevantly moderate. The reason might be that membrane anchored enzymes facilitate exportation of large quantity of fatty acids so the amount of fatty acid remaining in cytoplasm is limited. It is notable that C19 fatty acids only exist in cytoplasm because large molecular weight of C19 fatty acid prevents it from exportation and finally they are trapped in the cell. C19 fatty acids yield in ''Membrane Accelerator'' Group is even less than Group with diffusing cytoplasmic enzymes. We suppose it is caused by the high efficiency of TesA which leads to quick release of free fatty acid. | The fatty acids yields increase in sediment is relevantly moderate. The reason might be that membrane anchored enzymes facilitate exportation of large quantity of fatty acids so the amount of fatty acid remaining in cytoplasm is limited. It is notable that C19 fatty acids only exist in cytoplasm because large molecular weight of C19 fatty acid prevents it from exportation and finally they are trapped in the cell. C19 fatty acids yield in ''Membrane Accelerator'' Group is even less than Group with diffusing cytoplasmic enzymes. We suppose it is caused by the high efficiency of TesA which leads to quick release of free fatty acid. | ||
| Line 166: | Line 174: | ||
==Future Direction== | ==Future Direction== | ||
| + | [[File:12SJTU_Cyano.jpg|350px|right|thumb|Fig.13 ''Cyanobacteria'' under light microscope]] | ||
We haven't optimized experiment conditions with respect to strains, growth media, temperature etc., but the result has proved that ''Membrane Accelerator'' could accelerate biosynthetic pathway in ''E.coli'' marvelously. Moreover, changing the proportion of different enzymes in each enzyme assembly on membrane would promisingly enhance biosynthesis rate even more. In the near future, we expected to optimize those conditions to further improve the efficiency of ''Membrane Accelerator''. | We haven't optimized experiment conditions with respect to strains, growth media, temperature etc., but the result has proved that ''Membrane Accelerator'' could accelerate biosynthetic pathway in ''E.coli'' marvelously. Moreover, changing the proportion of different enzymes in each enzyme assembly on membrane would promisingly enhance biosynthesis rate even more. In the near future, we expected to optimize those conditions to further improve the efficiency of ''Membrane Accelerator''. | ||
Latest revision as of 03:54, 27 October 2012
| ||
|
 "
"