Team:SJTU-BioX-Shanghai/Project/project1.1
From 2012.igem.org
AleAlejandro (Talk | contribs) (→Antibiotics Test) |
AleAlejandro (Talk | contribs) (→Membrane Localization Test) |
||
| (69 intermediate revisions not shown) | |||
| Line 25: | Line 25: | ||
__NOTOC__ | __NOTOC__ | ||
<!----------------------------------------------------从这里开始写wiki---------------------------------> | <!----------------------------------------------------从这里开始写wiki---------------------------------> | ||
| - | + | =Membrane Localization= | |
| + | {{Template:12SJTU_part_summary_head}} | ||
| - | + | To make membrane into a scaffold, we must first make sure that our device is directed to inner membrane of ''E.coli''. To achieve this goal, we used native inner membrane protein of ''E.coli'' and a well-studied signaling sequence as basic components to direct fusion protein onto inner membrane. To justify this construction strategy, we constructed a novel testing fusion protein and conducted two tests to determine the localization of the engineered membrane protein. | |
| - | + | ||
| - | + | To express multiple large proteins at the same time in ''E.coli'', we reconstructed 3 compatible plasmids with araBAD promoter. | |
| - | All proteins | + | {{Template:12SJTU_part_summary_foot}} |
| - | [[Image:12SJTU-pACYC.png| | + | ==Reconstructed Vectors== |
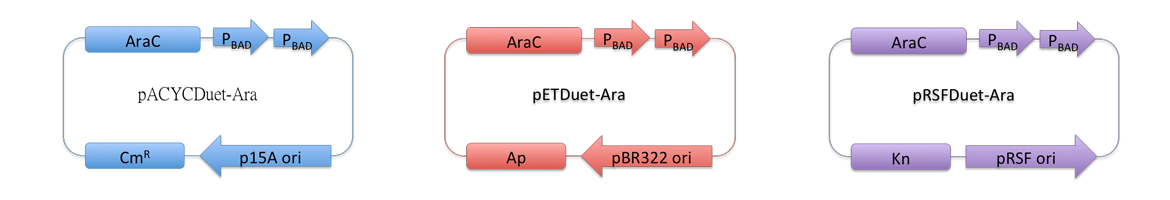
| - | ''Fig. 1 | + | Vectors used in the project for membrane protein expression are modified versions of pRSFDuet-1, pETDuet-1,pACYCDuet-1(''NOVAGEN''), which are originally regulated by T7 promoter. These plasmids can '''coexist''' in one cell. |
| - | + | ||
| - | + | Considering our multiple-enzyme system, it is necessary to use those compatible plasmids. However, T7 promoter is hard to be fine-tuned in ''E. coli''. To stress the advantage of the system, we recruited a weaker promoter, araBAD promoter, which could moderately response to a wide range of inducer (L-Arabinose) concentration. So we replaced T7 promoter in original plasmids with araBAD promoter (''Fig.1''). | |
| - | + | ||
| - | + | All proteins in our project are inserted into those three reconstructed vectors (pRSFDuet-Ara, pETDuet-Ara,pACYCDuet-Ara) and under control of araBAD promoter. | |
| - | + | [[Image:12SJTU-pACYC.png|700px|thumb|center|''Fig.1'' : Profile of reconstructed plasmids in ''Membrane Magic'' Project]] | |
| - | + | ==Membrane Protein Construction== | |
| - | + | ||
To construct membrane assemblies, we must make sure that our device is directed to inner membrane of ''E.coli''. | To construct membrane assemblies, we must make sure that our device is directed to inner membrane of ''E.coli''. | ||
| - | The first step is to choose an membrane anchor upon which other components could be built. To avoid toxicity caused by foreign membrane proteins, we chose ''phosphatidylglycerol:: prolipoprotein diacylglyceryl transferase'' (Lgt) as | + | The first step is to choose an membrane anchor upon which other components could be built. To avoid toxicity caused by foreign membrane proteins, we chose ''phosphatidylglycerol:: prolipoprotein diacylglyceryl transferase'' (Lgt) as core component of fusion protein. Lgt is an inner membrane protein of ''E.coli'' with seven transmembrane segments and has been successfully overexpressed in ''E. coli'' without causing harm to cells. |
| - | SsDsbA is the signal recognition particle (SRP)-dependent signaling sequence of DsbA. SsDsbA-tagged proteins are exported to the periplasm through the SRP pathway. With ssDsbA fused to the N-terminus, | + | SsDsbA is the signal recognition particle (SRP)-dependent signaling sequence of DsbA. SsDsbA-tagged proteins are exported to the periplasm through the SRP pathway. With ssDsbA fused to the N-terminus, fusion proteins with Lgt are expected to be anchored onto inner membrane of ''E.coli'' (''Fig.2'' ). |
| - | To justify the localization ability of fusion membrane proteins mentioned above, we constructed a novel fusion protein called '''BlaLG'''. β-lactamase is fused to the N-terminus of Lgt and GFP is fused to the C-terminus of Lgt. The fusion protein in under control of araBAD promoter. | + | Flexible linker FL3 is introduced between each crucial protein domain to ensure the proper function of them. |
| - | [[Image:12SJTU-project1-1.png|thumb| | + | |
| + | [[Image:12SJTU_proteinconstruction.jpg|thumb|700px|center|''Fig.2'' : Construction details of membrane assemblies]] | ||
| + | |||
| + | ==Membrane Localization Test== | ||
| + | To justify the localization ability of fusion membrane proteins mentioned above, we constructed a novel fusion protein called '''BlaLG'''([http://partsregistry.org/wiki/index.php?title=Part:BBa_K771401 BBa_K771401]). β-lactamase is fused to the N-terminus of Lgt and GFP is fused to the C-terminus of Lgt. The fusion protein in under control of araBAD promoter (''Fig.3'' ). | ||
| + | [[Image:12SJTU-project1-1.png|thumb|700px|center|''Fig.3'' : Details of fusion protein BlaLG for membrane localization]] | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
===Fluorescence Test=== | ===Fluorescence Test=== | ||
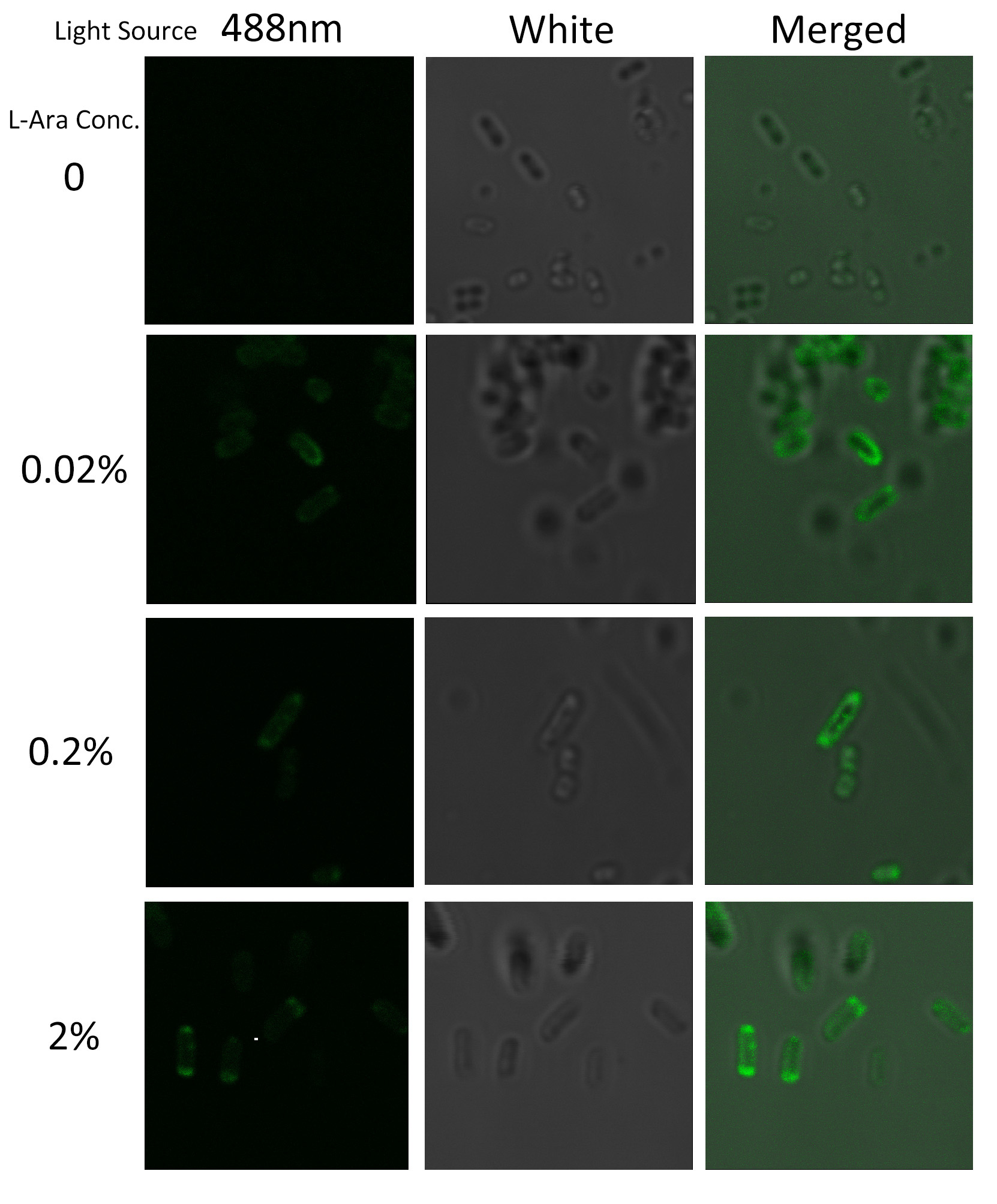
| - | To visualize the localization of | + | To visualize the localization of fusion protein BlaLG, we adopted fluorescence test. GFP fused to the C terminus of BlaLG enables us to have a closer look at where this fusion protein is localized (''Fig.3''). |
| - | + | Under laser confocal microscope, we can observe the location of green fluorescence, thus to confirm the exact subcellular localization of the fusion protein BlaLG. | |
[[Image:12SJTU_MLGFP.jpg|thumb|500px|center|''Fig.4'' :Bacteria carrying BlaLG induced at different concentration of L-arabinose.]] | [[Image:12SJTU_MLGFP.jpg|thumb|500px|center|''Fig.4'' :Bacteria carrying BlaLG induced at different concentration of L-arabinose.]] | ||
| - | It is observed that green fluorescence intensity of ''E.coli'' margin is higher than that of cytoplasm. ''Fig.4'' proves | + | It is observed that green fluorescence intensity of ''E.coli'' margin is higher than that of cytoplasm. ''Fig.4'' proves that BlaLG has been localized to membrane. |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
===Antibiotics Test=== | ===Antibiotics Test=== | ||
| - | In this section, we | + | In this section, we further tested the membrane localization ability of fusion membrane protein '''BlaLG''' by observing host cells' growth phenotype under different level of Ampicillin. Besides, we found the optimal inducer (L-arabinose) concentration for expressing membrane proteins. |
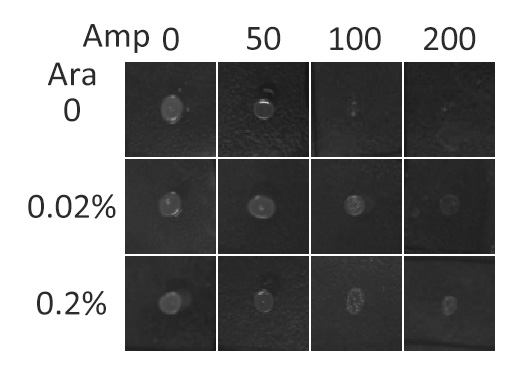
| - | β- | + | β-Lactamase,a bacterial enzyme which can confer Ampicillin resistance to its hosts only when it is in the periplasm. Note that N-terminus of Lgt faces the periplasm and C-terminus faces the cytoplasm (''Fig.3''). Hence, if BlaLG is correctly anchored to membrane, β-lactamase is expected to be functional and host cells should be able to grow on culture media containing ampicillin. |
| - | [[Image:12SJTU_AntiTest.jpg|thumb|400px|center|''Fig.5'' :Growth phenotypes of ''E. coli'' | + | [[Image:12SJTU_AntiTest.jpg|thumb|400px|center|''Fig.5'' :Growth phenotypes of ''E. coli'' expressing BlaLG on LB agar media with concentration of inducer L-arabinose from 0 to 0.2%. We increased the concentration of ampicillin from 0 to 200 (μg/ ml). Bacteria carrying BlaLG were able to grow at ampicillin concentration of 200 μg/ ml with induction]] |
| - | ''Fig.5'' showed that | + | ''Fig.5'' showed that BlaLG has been correctly localized to membrane. Besides, a very low L-arabinose concentration at 0.02% is already enough to induce sufficient amount of membrane protein. |
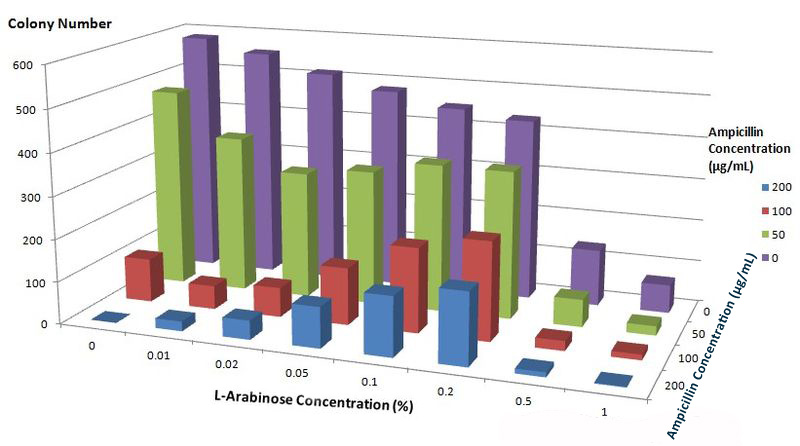
| + | To further characterize features of the Membrane Scaffold System and find optimal inducer concentration, we quantitatively tested the growth condition of ''E.coli'' carrying gene of BlaLG under different concentration of L-Arabinose and Ampicillin. We expected to see more colonies grew at high Ampicillin concentration if the inducer concentration is optimal. | ||
| - | |||
| + | [[Image:12SJTU_Antibioticstest2.jpg|thumb|600px|center|''Fig.6'' :Growth condition of ''E. coli'' cells expressing BlaLG on LB agar media with different concentration of L-arabinose and Ampicillin. Single colony is picked and cultivated at 37℃ until OD value reaches 0.7. Bacteria cultures are diluted by 1:100000 and coated onto plates containing graded concentration of L-Arabinose and Ampicillin. Growth condition is measured through counting colonies on plates.]] | ||
| - | + | The result apparently showed that at L-Arabinose concentration of 0.1% and 0.2%, more bacteria could grow on high concentration of Ampicllin. Thus, L-Arabinose concentration of 0.1% and 0.2% best suits single membrane protein expression in Project ''Membrane Magic''. | |
| - | + | ||
| - | The result apparently showed that at L-Arabinose concentration of 0.1% and 0.2%, more bacteria could grow on high concentration of Ampicllin. Thus, L-Arabinose concentration of 0.1% and 0.2 best suits membrane protein expression in Project ''Membrane Magic''. | + | |
<br> | <br> | ||
<br> | <br> | ||
<br> | <br> | ||
| + | |||
| + | === Conclusion === | ||
| + | The result proved that fusion membrane protein with construction strategy shown in ''Fig. 2'' could be effectively localized to inner membrane of ''E.coli''. Thus membrane could readily be used as scaffold carrying various enzymes. Based on membrane scaffold, we tried to build two universal devices: [https://2012.igem.org/Team:SJTU-BioX-Shanghai/Project/project1.2 Membrane Accelerator] and [https://2012.igem.org/Team:SJTU-BioX-Shanghai/Project/project1.3 Membrane Rudder]. | ||
| + | |||
<br> | <br> | ||
<br> | <br> | ||
| - | + | ------------------------------------------------------------------------ | |
| + | |||
| + | ==Reference== | ||
1.Pailler, J., W. Aucher, et al. (2012). "Phosphatidylglycerol:: prolipoprotein diacylglyceryl transferase (Lgt) of Escherichia coli has seven transmembrane segments, and its essential residues are embedded in the membrane." Journal of bacteriology 194(9): 2142-2151. | 1.Pailler, J., W. Aucher, et al. (2012). "Phosphatidylglycerol:: prolipoprotein diacylglyceryl transferase (Lgt) of Escherichia coli has seven transmembrane segments, and its essential residues are embedded in the membrane." Journal of bacteriology 194(9): 2142-2151. | ||
Latest revision as of 02:57, 27 October 2012
| ||
|
 "
"