Team:Tokyo Tech/Projects/PHAs/index.htm
From 2012.igem.org
| Line 4: | Line 4: | ||
<div class="whitebox"> | <div class="whitebox"> | ||
<div id="tokyotech" style=" font:bold ;left ; font-size: 50px; color: #1E90FF; padding: 10px;"> | <div id="tokyotech" style=" font:bold ;left ; font-size: 50px; color: #1E90FF; padding: 10px;"> | ||
| - | + | PHAs Production </div> | |
</div class="whitebox"> | </div class="whitebox"> | ||
<div class="whitebox"> | <div class="whitebox"> | ||
<div id="tokyotech" style=" font:Arial ;left ; font-size: 15px; color: #000000; padding: 30px;"> | <div id="tokyotech" style=" font:Arial ;left ; font-size: 15px; color: #000000; padding: 30px;"> | ||
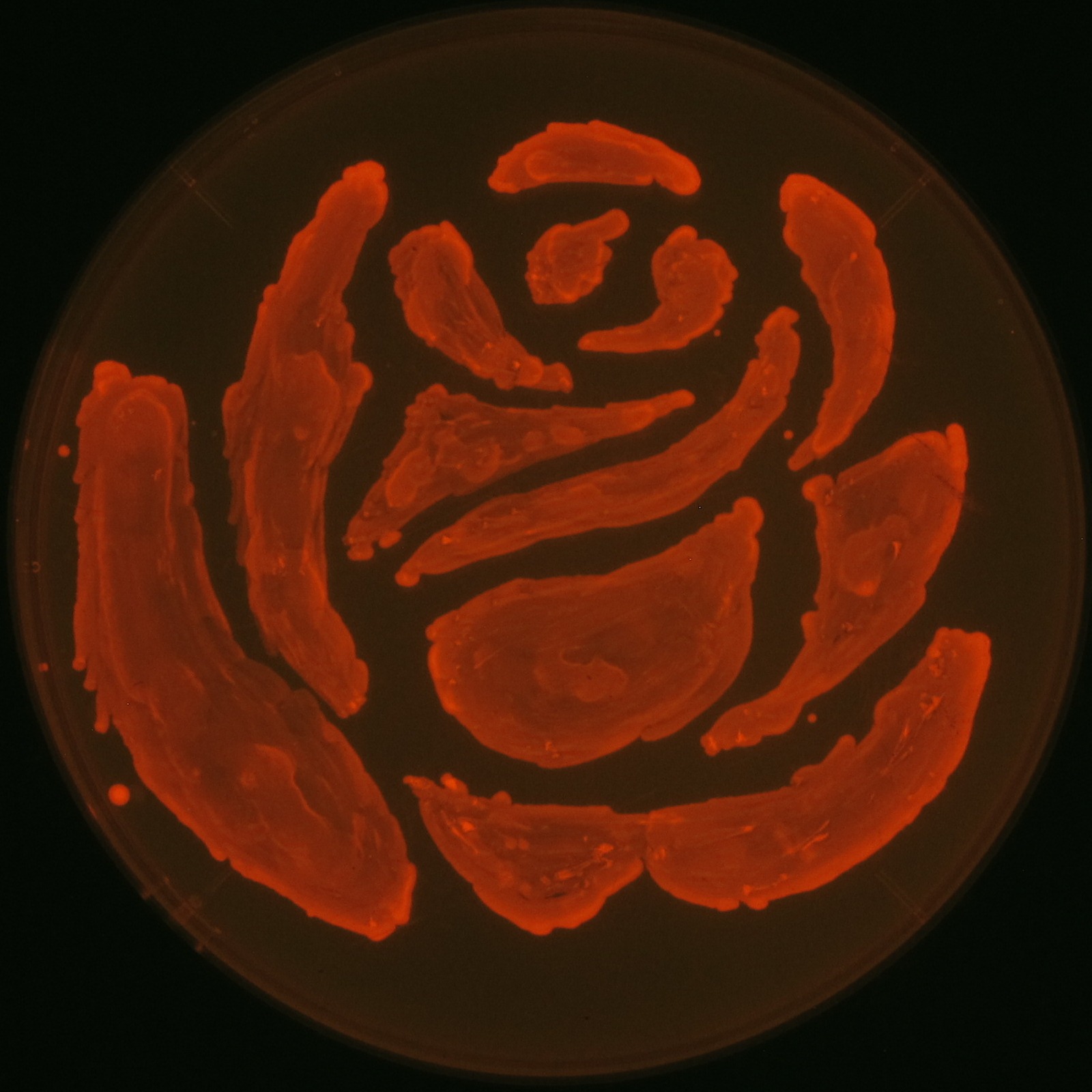
| - | [[File:tokyotech | + | [[File:tokyotech PHA make rose.png|400px|thumb|right|Fig2-2-1-1, Rose silhouette on the LB agar plate containing Nile red.]] |
__TOC__ | __TOC__ | ||
<br> | <br> | ||
| Line 14: | Line 14: | ||
1.</div> | 1.</div> | ||
=Achivement= | =Achivement= | ||
| - | We made a new biobrick part and succeeded in synthesizing Polyhydroxyalkanoates( | + | We made a new biobrick part and succeeded in synthesizing Polyhydroxyalkanoates(PHAs). This is the first Biobrick part to synthesize PHAs. |
| - | In our project, we also drew rose silhouette to produce the balcony scene of “Romeo and Juliet” by the synthesis of | + | In our project, we also drew rose silhouette to produce the balcony scene of “Romeo and Juliet” by the synthesis of PHAs. |
<br><br> | <br><br> | ||
<div id="tokyotech" style=" font:bold ;left ; font-size: 30px; color: #0000FF; padding: 2px;"> | <div id="tokyotech" style=" font:bold ;left ; font-size: 30px; color: #0000FF; padding: 2px;"> | ||
2.</div> | 2.</div> | ||
| - | =What is | + | =What is PHA?= |
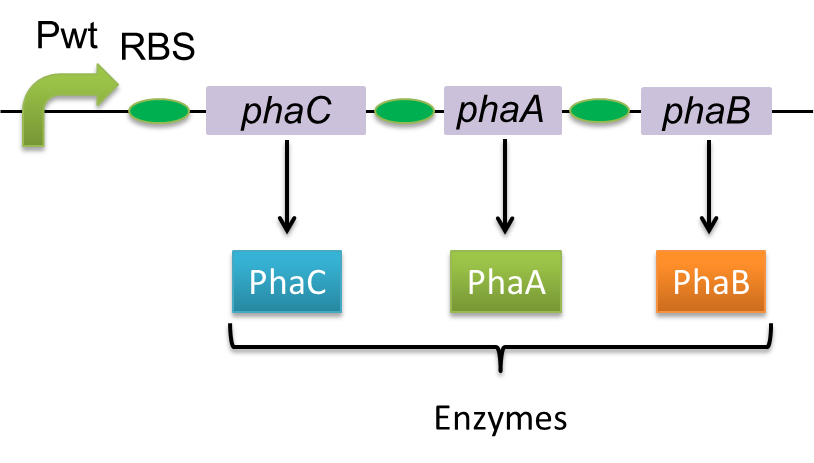
| - | Polyhydroxyalkanoates( | + | Polyhydroxyalkanoates(PHAs) are biological polyester synthesized by a wide range of bacteria, and can be produced by fermentation from renewable carbon sources such as sugars and vegetable oils. These polyesters are biodegradable thermoplastics and elastomers, which exhibit interesting material properties. PHAs are also a kind of bio plastics, which can be biodegraded a lot faster than fossil-fuel plastics in the environment. Poly-3-hydroxybutyrate, P(3HB) is the most common type of PHAs. P(3HB) is synthesized by the enzymes coded in the gene of PHA synthesis (phaC1-A-B1) from Ralstonia eutropha H16. |
| - | [[File:tokyotech | + | [[File:tokyotech PHA whatsPHA.png|300px|thumb|left|Fig2-2-2-1, Gene of PHA synthesis (phaC1-A-B1) from Ralstonia eutropha H16.]] |
<br><br> | <br><br> | ||
Poly-3-hydroxybutyrate, P(3HB) is synthesized by three enzymes. | Poly-3-hydroxybutyrate, P(3HB) is synthesized by three enzymes. | ||
| Line 29: | Line 29: | ||
| - | The A gene encodes for the 393 amino acids protein, 3-ketothiolase ( | + | The A gene encodes for the 393 amino acids protein, 3-ketothiolase (PhaA) |
| - | The B gene encodes for the 246 amino acids protein, acetoacetyl-CoA reductase ( | + | The B gene encodes for the 246 amino acids protein, acetoacetyl-CoA reductase (PhaB) |
| - | The C gene encodes for the 589 amino acids protein, | + | The C gene encodes for the 589 amino acids protein, PHA Synthase (PhaC) |
<br><br><br><br><br> | <br><br><br><br><br> | ||
| - | [[File:tokyotech | + | [[File:tokyotech PHA whatsPHA2.png|150px|thumb|left|Fig2-2-2-2, synthesis mechanism of P(3HB)]] |
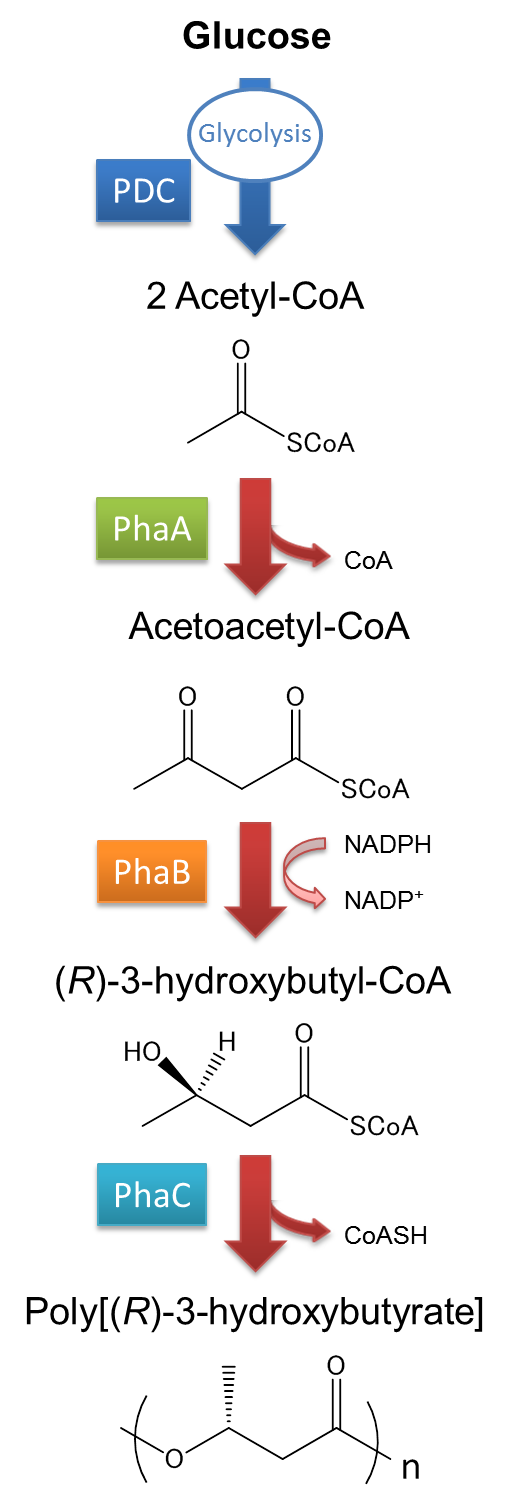
| - | The pathway and regulation of Poly[(R)-3-hydroxybutyrate], P(3HB) synthesis in | + | The pathway and regulation of Poly[(R)-3-hydroxybutyrate], P(3HB) synthesis in Ralstonia eutropha H16 is shown in Fig2-2-2-2. Pyruvic acid is metabolized from glucose by glycolysis, and pyruvate dehydrogenase complex (PDC) transforms pyruvic acid into acetyl-CoA. At first, two molecules of acetyl-CoA are ligated to one molecule acetoacetyl-CoA by the action of 3-ketothiolase (coded in phaA). Acetoacetyl-CoA is transformed into (R)-3-hydroxybutyl-CoA by NADPH dependent acetoacetyl-CoA reductase (coded in phaB). P(3HB) is then synthesized by the polymerization of (R)-3-hydroxybutyryl-CoA by the action of PHA synthase (PhaC).([[#Reference|[1][2]]] |
) | ) | ||
| Line 46: | Line 46: | ||
3.</div> | 3.</div> | ||
| - | =Construction of | + | =Construction of phaC1-A-B1 in Biobrick format= |
| - | In this study, we constructed a part containing | + | In this study, we constructed a part containing phaC1-A-B1 in Biobrick format([http://partsregistry.org/wiki/index.php?title=Part:BBa_K934001 BBa_K934001]).[[https://2012.igem.org/Team:Tokyo_Tech/Projects/PHAs/detail/index.htm#Construction_of_pha-C1-A-B1_in_Biobrick_format Construction of pha-C1-A-B1 in Biobrick format]] |
| - | This is the first Biobrick part which worked as expected though some teams had tried to synthesize | + | This is the first Biobrick part which worked as expected though some teams had tried to synthesize PHAs in the past iGEM.[[https://2012.igem.org/Team:Tokyo_Tech/Projects/PHAs/detail/index.htm#Production_trial_of_PHAs_by_past_teams Production trial of PHAs by past teams]] |
<br><br><br><br><br><br><br> | <br><br><br><br><br><br><br> | ||
| Line 57: | Line 57: | ||
=PHB production by <I>E.coli</I> & Confirmation of PHB= | =PHB production by <I>E.coli</I> & Confirmation of PHB= | ||
| - | To synthesize PHB by <I>E.coli</I>, we transformed <I>E.coli</I> JM109 with the constructed | + | To synthesize PHB by <I>E.coli</I>, we transformed <I>E.coli</I> JM109 with the constructed phaC1-A-B1 part on pSB1C3 ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K934001 BBa_K934001]). <I>E.coli</I> JM109 is used to synthesize PHB, because it tends to have a high density accumulation of PHB([[#Reference|[5]]] |
). As a negative control, we transformed <I>E.coli</I> JM109 with PlasI-gfp on pSB1C3. | ). As a negative control, we transformed <I>E.coli</I> JM109 with PlasI-gfp on pSB1C3. | ||
==4-1 Confirmation of PHB synthesized on colonies== | ==4-1 Confirmation of PHB synthesized on colonies== | ||
| - | We observed the accumulation of PHB in the <I>E.coli</I> colonies on Nile red positive medium under UV. Nile red has been widely used to stain colonies and distinguish between | + | We observed the accumulation of PHB in the <I>E.coli</I> colonies on Nile red positive medium under UV. Nile red has been widely used to stain colonies and distinguish between PHA-accumulating and non-accumulating colonies. Nile red in the agar medium doesn’t affect the growth of the cells, and the accumulation of PHAs in the colonies can be directly monitored([[#Reference|[3][4][5]]] |
). We cultured the transformant on LB agar medium plates with Nile red. After several days, colonies storing PHB were stained orange by Nile red when observed under UV. This result indicates that transformant synthesized and stored PHB. | ). We cultured the transformant on LB agar medium plates with Nile red. After several days, colonies storing PHB were stained orange by Nile red when observed under UV. This result indicates that transformant synthesized and stored PHB. | ||
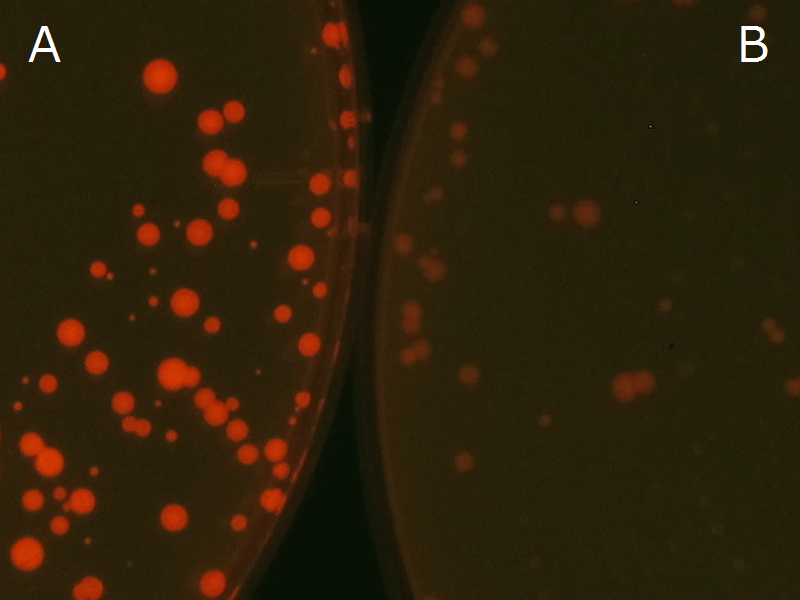
Fig2-2-4-1 is the photographs of <I>E.coli</I> colonies on Nile red positive medium taken under UV. The orange colonies in Fig2-2-4-1A show that the accumulated PHB in cells was stained by Nile red. This result indicates that part [http://partsregistry.org/wiki/index.php?title=Part:BBa_K934001 BBa_K934001] synthesized PHB. Fig2-2-4-1B is the photograph of negative control cells. In this figure we observed that there were no remarkable colored colonies. Fig2-2-4-1-2 shows the difference between cells storing PHB and those not storing PHB on one plate. The cells in blue rectangle area are the cells with PHB synthesis gene and the cells in green rectangle area are the cells with PlasI-gfp gene as a negative control. Using the cells storing PHB, we drew a rose silhouette on the LB agar plate containing Nile red (Fig2-2-4-1-3).[[https://2012.igem.org/Team:Tokyo_Tech/Projects/PHAs/detail/index.htm#A_.PHB_production_on_colonies_and_preparation_before_confirmation_with_Nile_red_under_UV Protocol]] | Fig2-2-4-1 is the photographs of <I>E.coli</I> colonies on Nile red positive medium taken under UV. The orange colonies in Fig2-2-4-1A show that the accumulated PHB in cells was stained by Nile red. This result indicates that part [http://partsregistry.org/wiki/index.php?title=Part:BBa_K934001 BBa_K934001] synthesized PHB. Fig2-2-4-1B is the photograph of negative control cells. In this figure we observed that there were no remarkable colored colonies. Fig2-2-4-1-2 shows the difference between cells storing PHB and those not storing PHB on one plate. The cells in blue rectangle area are the cells with PHB synthesis gene and the cells in green rectangle area are the cells with PlasI-gfp gene as a negative control. Using the cells storing PHB, we drew a rose silhouette on the LB agar plate containing Nile red (Fig2-2-4-1-3).[[https://2012.igem.org/Team:Tokyo_Tech/Projects/PHAs/detail/index.htm#A_.PHB_production_on_colonies_and_preparation_before_confirmation_with_Nile_red_under_UV Protocol]] | ||
| Line 76: | Line 76: | ||
To confirm the accumulation condition of PHB in <I>E.coli</I> with a microscope, we stained the PHB with Nile blue A reagent. Nile blue A is also used to detect the existence of PHB and has no toxicity to the cells([[#Reference|[5]]]). Before the observation, we stained the dried cells with Nile blue A solution. We then took photographs of the sample under fluorescence microscope. | To confirm the accumulation condition of PHB in <I>E.coli</I> with a microscope, we stained the PHB with Nile blue A reagent. Nile blue A is also used to detect the existence of PHB and has no toxicity to the cells([[#Reference|[5]]]). Before the observation, we stained the dried cells with Nile blue A solution. We then took photographs of the sample under fluorescence microscope. | ||
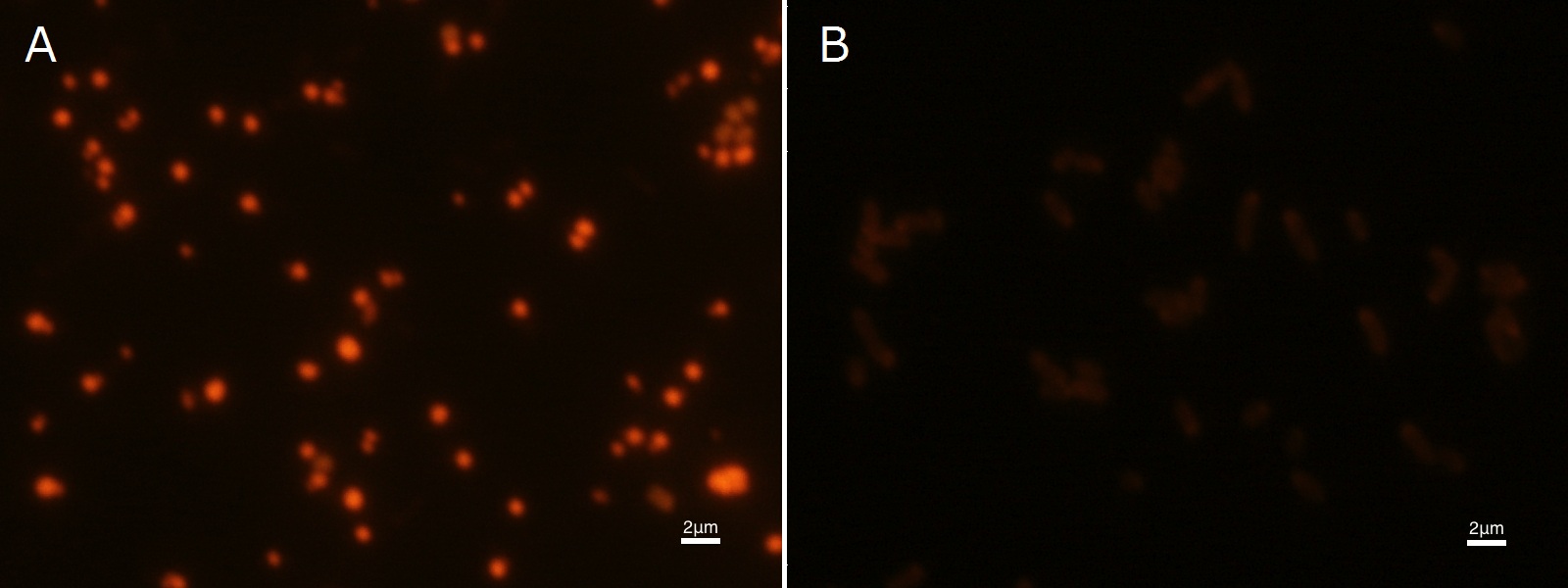
| - | Fig2-2-4-2-1 is the photograph of dried <I>E.coli</I> (with | + | Fig2-2-4-2-1 is the photograph of dried <I>E.coli</I> (with phaC1-A-B1 gene) cells dyed with Nile blue A solution taken by fluorescence microscope. The fluorescent areas in Fig2-2-4-2-1A are the accumulated PHB in the cells. This result also indicates that part [http://partsregistry.org/wiki/index.php?title=Part:BBa_K934001 BBa_K934001] synthesized PHB. In the photograph of negative control (Fig2-2-4-2-1B), no remarkable fluorescent area was observed.[[https://2012.igem.org/Team:Tokyo_Tech/Projects/PHAs/detail/index.htm#B.PHB_production_in_cells_and_preparation_before_the_confirmation_with_Nile_blue_A Protocol]] |
[[File:tokyotech PHA Nileblue1.png|500px|thumb|center| | [[File:tokyotech PHA Nileblue1.png|500px|thumb|center| | ||
| Line 87: | Line 87: | ||
=Possible Synbio research area by using our achievement= | =Possible Synbio research area by using our achievement= | ||
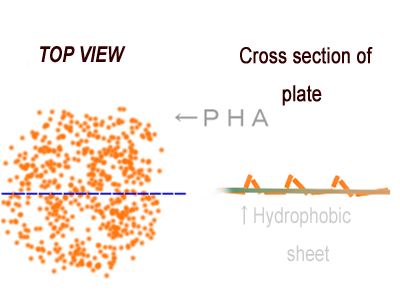
| - | [[File:tokyotech PHA perspective.png|200px|thumb|right|Fig2-2-5-1, | + | [[File:tokyotech PHA perspective.png|200px|thumb|right|Fig2-2-5-1, PHA gene expression spatially manipulated]] |
| - | The achievement of our project | + | The achievement of our project “PHAs Production” is that we registered available PHA synthetic gene in Biobrick parts. |
| - | We can control the expression of the | + | We can control the expression of the PHA synthetic gene spatially by using combination of Biobrick parts. What we want to claim as an example of the spatial manipulation of gene expression is water-repellent. A stronger water-repellent requires hydrophobicity as well as the increase in real surface area that can be achieved as ruggedness of PHA adsorbed on particular surface. If we can control the expression of the PHA synthetic gene spatially by using genetic parts which are registered in Biobrick parts, the application of a super water-repellent sheet will become available. We note this as to the future prospects of our project. |
<br> | <br> | ||
<div id="tokyotech" style=" font:bold ;left ; font-size: 30px; color: #0000FF; padding: 2px;"> | <div id="tokyotech" style=" font:bold ;left ; font-size: 30px; color: #0000FF; padding: 2px;"> | ||
| Line 99: | Line 99: | ||
[1] Jumiarti Agus, Altered expression of polyhydroxyalkanoate synthase gene and its effect on poly[(R)-3-hydroxybutyrate] synthesis in recombinant Escherichia coli, Polymer Degradation and Stability(2006) 91:1645-1650 | [1] Jumiarti Agus, Altered expression of polyhydroxyalkanoate synthase gene and its effect on poly[(R)-3-hydroxybutyrate] synthesis in recombinant Escherichia coli, Polymer Degradation and Stability(2006) 91:1645-1650 | ||
| - | [2] Joanne Stubbe and Jiamin Tian, Polyhydroxyalkanoate ( | + | [2] Joanne Stubbe and Jiamin Tian, Polyhydroxyalkanoate (PHA) homeostasis: the role of the PHA synthase, 2003, Nat. Prod. Rep.,20, 445–457. |
[3] Stanley D. Fowler and Phillip Greenspan, Application of Nile red, a fluorescent hydrophobic probe, for the detection of neutral lipid deposits in tissue sections, Histochemistry & Cytochemistry(1985), vol 33.No 8, 833-836 | [3] Stanley D. Fowler and Phillip Greenspan, Application of Nile red, a fluorescent hydrophobic probe, for the detection of neutral lipid deposits in tissue sections, Histochemistry & Cytochemistry(1985), vol 33.No 8, 833-836 | ||
Revision as of 04:30, 21 October 2012
Contents |
Achivement
We made a new biobrick part and succeeded in synthesizing Polyhydroxyalkanoates(PHAs). This is the first Biobrick part to synthesize PHAs.
In our project, we also drew rose silhouette to produce the balcony scene of “Romeo and Juliet” by the synthesis of PHAs.
What is PHA?
Polyhydroxyalkanoates(PHAs) are biological polyester synthesized by a wide range of bacteria, and can be produced by fermentation from renewable carbon sources such as sugars and vegetable oils. These polyesters are biodegradable thermoplastics and elastomers, which exhibit interesting material properties. PHAs are also a kind of bio plastics, which can be biodegraded a lot faster than fossil-fuel plastics in the environment. Poly-3-hydroxybutyrate, P(3HB) is the most common type of PHAs. P(3HB) is synthesized by the enzymes coded in the gene of PHA synthesis (phaC1-A-B1) from Ralstonia eutropha H16.
Poly-3-hydroxybutyrate, P(3HB) is synthesized by three enzymes.
The A gene encodes for the 393 amino acids protein, 3-ketothiolase (PhaA)
The B gene encodes for the 246 amino acids protein, acetoacetyl-CoA reductase (PhaB)
The C gene encodes for the 589 amino acids protein, PHA Synthase (PhaC)
The pathway and regulation of Poly[(R)-3-hydroxybutyrate], P(3HB) synthesis in Ralstonia eutropha H16 is shown in Fig2-2-2-2. Pyruvic acid is metabolized from glucose by glycolysis, and pyruvate dehydrogenase complex (PDC) transforms pyruvic acid into acetyl-CoA. At first, two molecules of acetyl-CoA are ligated to one molecule acetoacetyl-CoA by the action of 3-ketothiolase (coded in phaA). Acetoacetyl-CoA is transformed into (R)-3-hydroxybutyl-CoA by NADPH dependent acetoacetyl-CoA reductase (coded in phaB). P(3HB) is then synthesized by the polymerization of (R)-3-hydroxybutyryl-CoA by the action of PHA synthase (PhaC).([1][2]
)
Construction of phaC1-A-B1 in Biobrick format
In this study, we constructed a part containing phaC1-A-B1 in Biobrick format([http://partsregistry.org/wiki/index.php?title=Part:BBa_K934001 BBa_K934001]).[Construction of pha-C1-A-B1 in Biobrick format]
This is the first Biobrick part which worked as expected though some teams had tried to synthesize PHAs in the past iGEM.[Production trial of PHAs by past teams]
PHB production by E.coli & Confirmation of PHB
To synthesize PHB by E.coli, we transformed E.coli JM109 with the constructed phaC1-A-B1 part on pSB1C3 ([http://partsregistry.org/wiki/index.php?title=Part:BBa_K934001 BBa_K934001]). E.coli JM109 is used to synthesize PHB, because it tends to have a high density accumulation of PHB([5] ). As a negative control, we transformed E.coli JM109 with PlasI-gfp on pSB1C3.
4-1 Confirmation of PHB synthesized on colonies
We observed the accumulation of PHB in the E.coli colonies on Nile red positive medium under UV. Nile red has been widely used to stain colonies and distinguish between PHA-accumulating and non-accumulating colonies. Nile red in the agar medium doesn’t affect the growth of the cells, and the accumulation of PHAs in the colonies can be directly monitored([3][4][5] ). We cultured the transformant on LB agar medium plates with Nile red. After several days, colonies storing PHB were stained orange by Nile red when observed under UV. This result indicates that transformant synthesized and stored PHB. Fig2-2-4-1 is the photographs of E.coli colonies on Nile red positive medium taken under UV. The orange colonies in Fig2-2-4-1A show that the accumulated PHB in cells was stained by Nile red. This result indicates that part [http://partsregistry.org/wiki/index.php?title=Part:BBa_K934001 BBa_K934001] synthesized PHB. Fig2-2-4-1B is the photograph of negative control cells. In this figure we observed that there were no remarkable colored colonies. Fig2-2-4-1-2 shows the difference between cells storing PHB and those not storing PHB on one plate. The cells in blue rectangle area are the cells with PHB synthesis gene and the cells in green rectangle area are the cells with PlasI-gfp gene as a negative control. Using the cells storing PHB, we drew a rose silhouette on the LB agar plate containing Nile red (Fig2-2-4-1-3).[Protocol]
4-2 Confirmation of PHB accumulated in cells
To confirm the accumulation condition of PHB in E.coli with a microscope, we stained the PHB with Nile blue A reagent. Nile blue A is also used to detect the existence of PHB and has no toxicity to the cells([5]). Before the observation, we stained the dried cells with Nile blue A solution. We then took photographs of the sample under fluorescence microscope. Fig2-2-4-2-1 is the photograph of dried E.coli (with phaC1-A-B1 gene) cells dyed with Nile blue A solution taken by fluorescence microscope. The fluorescent areas in Fig2-2-4-2-1A are the accumulated PHB in the cells. This result also indicates that part [http://partsregistry.org/wiki/index.php?title=Part:BBa_K934001 BBa_K934001] synthesized PHB. In the photograph of negative control (Fig2-2-4-2-1B), no remarkable fluorescent area was observed.[Protocol]
Possible Synbio research area by using our achievement
The achievement of our project “PHAs Production” is that we registered available PHA synthetic gene in Biobrick parts.
We can control the expression of the PHA synthetic gene spatially by using combination of Biobrick parts. What we want to claim as an example of the spatial manipulation of gene expression is water-repellent. A stronger water-repellent requires hydrophobicity as well as the increase in real surface area that can be achieved as ruggedness of PHA adsorbed on particular surface. If we can control the expression of the PHA synthetic gene spatially by using genetic parts which are registered in Biobrick parts, the application of a super water-repellent sheet will become available. We note this as to the future prospects of our project.
Reference
[1] Jumiarti Agus, Altered expression of polyhydroxyalkanoate synthase gene and its effect on poly[(R)-3-hydroxybutyrate] synthesis in recombinant Escherichia coli, Polymer Degradation and Stability(2006) 91:1645-1650
[2] Joanne Stubbe and Jiamin Tian, Polyhydroxyalkanoate (PHA) homeostasis: the role of the PHA synthase, 2003, Nat. Prod. Rep.,20, 445–457.
[3] Stanley D. Fowler and Phillip Greenspan, Application of Nile red, a fluorescent hydrophobic probe, for the detection of neutral lipid deposits in tissue sections, Histochemistry & Cytochemistry(1985), vol 33.No 8, 833-836
[4] Pinzon NM, Nile red detection of bacterial hydrocarbons and ketones in a high-throughput format, mBio (2011),vol 2. issue 4.e-00109-11
[5] Patricia Spiekermann, A sensitive, viable-colony staining method using Nile red for direct screening of bacteria that accumulate polyhydroxyalkanoic acids and other lipid storage compounds, Arch Microbiol (1999), 171:73–80
[6] Vladimir K. Vanag, Cross-diffusion and pattern formation in reaction–diffusion systems, Physical Chemistry Chemical Physics(2009), vol 11.897-912 "
"