Team:Minnesota/Project/UV Absorption
From 2012.igem.org
(Difference between revisions)
m |
|||
| (6 intermediate revisions not shown) | |||
| Line 132: | Line 132: | ||
<div id="MainBoxContent" style="background-color:white;"> | <div id="MainBoxContent" style="background-color:white;"> | ||
| - | <div style="position:absolute;top:0px;margin-left:50px; width:600px; height:450px; overflow-y:scroll; text-align:left;"> <!---START HERE---> | + | <div style="position:absolute;top:0px;margin-left:50px; width:600px; height:450px; overflow-y:scroll; text-align:left;overflow-x:hidden;"> <!---START HERE---> |
<h1>Synthesizing UV-Protective Compounds in Bacteria</h1><br> | <h1>Synthesizing UV-Protective Compounds in Bacteria</h1><br> | ||
| Line 144: | Line 144: | ||
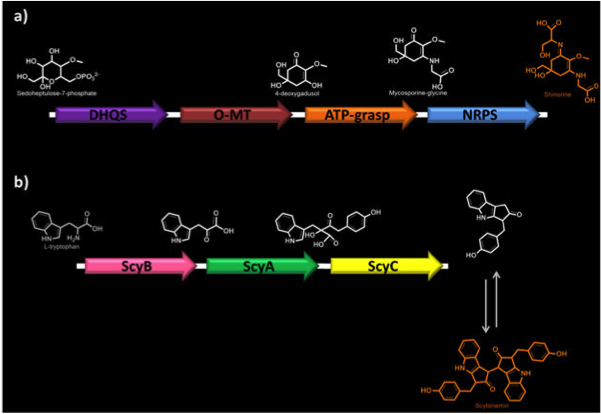
Our research has focused on two novel biosynthetic pathways found in two distinct algal species. A pathway ending in the production of two UV-protective compounds, shinorine and mycosporine-glycine, was cloned from <i>Anabaena varibalis</i>. A second pathway leading to the production of the unrelated UV-screening compound scytonemin was cloned from <i>Nostoc punctiforme</i>. Our objective is to develop novel and effective production platforms for these compounds, some of which are currently used in expensive sunscreens and lotions. These compounds are used both for the UV-absorptive properties as well as their role as potent antioxidants.</p> | Our research has focused on two novel biosynthetic pathways found in two distinct algal species. A pathway ending in the production of two UV-protective compounds, shinorine and mycosporine-glycine, was cloned from <i>Anabaena varibalis</i>. A second pathway leading to the production of the unrelated UV-screening compound scytonemin was cloned from <i>Nostoc punctiforme</i>. Our objective is to develop novel and effective production platforms for these compounds, some of which are currently used in expensive sunscreens and lotions. These compounds are used both for the UV-absorptive properties as well as their role as potent antioxidants.</p> | ||
| - | < | + | <img src="http://i1158.photobucket.com/albums/p607/iGEM_MN/1Bac.png"> |
| - | + | ||
| - | + | ||
| - | + | ||
<p><b>Figure 1. Biosynthetic pathways of MAAs.</b> a) Shinorine biosynthesis. DHQS, dehydroquinate synthase; O-MT, O-methyltransferase; ATP-grasp; NRPS, non-ribosomal peptide synthase. b) Scytonemin biosynthesis. ScyA (NpR1276), acetolactate synthetase; ScyB (NpR1275), leucine dehydrogenase; ScyC (NpR1274), protein of unknown function.</p> | <p><b>Figure 1. Biosynthetic pathways of MAAs.</b> a) Shinorine biosynthesis. DHQS, dehydroquinate synthase; O-MT, O-methyltransferase; ATP-grasp; NRPS, non-ribosomal peptide synthase. b) Scytonemin biosynthesis. ScyA (NpR1276), acetolactate synthetase; ScyB (NpR1275), leucine dehydrogenase; ScyC (NpR1274), protein of unknown function.</p> | ||
| Line 174: | Line 171: | ||
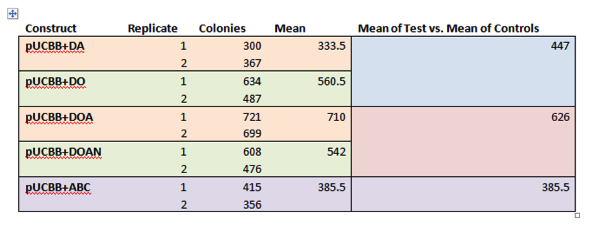
<p><b>Results</b><br> | <p><b>Results</b><br> | ||
| - | < | + | <img src="http://i1158.photobucket.com/albums/p607/iGEM_MN/2_Bac.png"> |
| + | |||
<p><b>Figure 2. HPLC analysis of E. coli expressing MAA biosynthetic genes.</b> Cell extracts of E. coli transformed with +DOA (DHQS, O-methyl transferase, and ATP-grasp) and +DOAN (DOA, and NRPS) showed production and 4-deoxygadusol (a) and mycosporine-glycine (b). Shinorine was not detected in the +DOAN expressing cells. Samples were compared against a +DA negative control (this construct lacks the ability to produce the 4-deoxygadusol intermediate). Cells expressing +ABC (ScyA, ScyB, ScyC) did not show scytonemin production. Data was compared with previous results from Balskus and Walsh, as a standard could not be obtained. </p> | <p><b>Figure 2. HPLC analysis of E. coli expressing MAA biosynthetic genes.</b> Cell extracts of E. coli transformed with +DOA (DHQS, O-methyl transferase, and ATP-grasp) and +DOAN (DOA, and NRPS) showed production and 4-deoxygadusol (a) and mycosporine-glycine (b). Shinorine was not detected in the +DOAN expressing cells. Samples were compared against a +DA negative control (this construct lacks the ability to produce the 4-deoxygadusol intermediate). Cells expressing +ABC (ScyA, ScyB, ScyC) did not show scytonemin production. Data was compared with previous results from Balskus and Walsh, as a standard could not be obtained. </p> | ||
| - | + | <img src="http://i1158.photobucket.com/albums/p607/iGEM_MN/3Bac.png"> | |
<p><b>Figure 3. UV sensitivity screens of E. coli cultures producing MAAs.</b> Preliminary results for the secondary screen showed increased survival in cells producing mycosporine-glycine when compared with +DO and +DA negative controls. Cells expressing +ABC did not show increased survivability.</p> | <p><b>Figure 3. UV sensitivity screens of E. coli cultures producing MAAs.</b> Preliminary results for the secondary screen showed increased survival in cells producing mycosporine-glycine when compared with +DO and +DA negative controls. Cells expressing +ABC did not show increased survivability.</p> | ||
| - | + | <img src="http://i1158.photobucket.com/albums/p607/iGEM_MN/cellimages.jpg"> | |
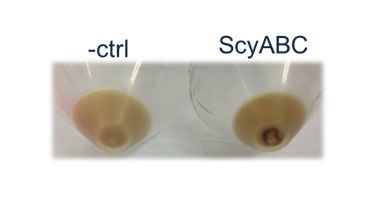
<p><b>Figure 4. Visual inspection of E. coli cells containing the scytonemin pathway.</b> Although HPLC and growth tests were negative, cells expressing +ABC were seen to be expressing a deep brown product that could easily be visualized (below). The control is a cell culture expressing a +DA plasmid vector. </p> | <p><b>Figure 4. Visual inspection of E. coli cells containing the scytonemin pathway.</b> Although HPLC and growth tests were negative, cells expressing +ABC were seen to be expressing a deep brown product that could easily be visualized (below). The control is a cell culture expressing a +DA plasmid vector. </p> | ||
Latest revision as of 03:58, 4 October 2012

Like us on FB and follow us on Twitter!
 "
"