Team:Cornell/Notebook/Wetlab
From 2012.igem.org
(→Bootcamp) |
(→June) |
||
| Line 17: | Line 17: | ||
::[[File:Cornell2012_0621_Gibson-entire-plasmid.jpg|600px]] | ::[[File:Cornell2012_0621_Gibson-entire-plasmid.jpg|600px]] | ||
| - | |||
==June 24th-30th== | ==June 24th-30th== | ||
Revision as of 19:52, 2 October 2012
Contents
|
Overview
We began the summer by holding a synthetic biology bootcamp in the DeLisa Lab. The purpose of this bootcamp was both to introduce new members to techniques in molecular biology and to get a running start on the cloning work for our project. During bootcamp, we successfully constructed both versions of our arsenic reporter, and attempted a Gibson assembly of a naphthalene-degrading plasmid.
In late June, we transitioned from bootcamp to our permanent bench space in Dr. Archer’s lab in Weill Hall. After spending a few weeks setting up the lab space troubleshooting general issues, we successfully constructed both versions of our salicylate reporter and began an alternative approach to construct a plasmid with a naphthalene-degrading (nah) operon. In parallel, we realized that electroporation efficiency for Shewanella transformation is less than optimal—to say the least. However, we were able to conjugate our constructs into Shewanella using a protocol provided by Dr. Gralnick.
As we transitioned into the fall semester in late August, wetlab work was divided into subprojects that could be accomplished in parallel. Subproject leaders independently worked on (1) characterizing our engineered Shewanella strains using reactors in the Angenent lab, (2) characterizing inducible promoters via qPCR of mtrB transcript in response to analyte, (3) characterizing inducible promoters in Shewanella via fluorescent reporters,(4) appending His-tags to MtrB in order to perform immunoassays, (5) performing site-directed mutagenesis on the nah operon to delete BioBrick cutsites, (6) constructing our final naphthalene-degrading plasmid to be conjugated into Shewanella, and (7) confirming that strains carrying the naphthalene-degrading plasmid can actually eat naphthalene.
Bootcamp
From June 12th-22nd, the DeLisa lab kindly hosted a synthetic biology ‘bootcamp’ for our team members. Dr. Didi Waraho assisted in the instruction of new team members, while Taylor Stevenson helped more experienced members get acquainted with the Gibson assembly method.
Didi’s group worked on the construction of our arsenic reporter plasmids (now referred to as p14k and p16k). This work involved PCR steps to append non-BioBrick cutsites (AscI and BamHI) to an existing mtrB BioBrick (BBa_K098994) and an arsenic-sensing region (BBa_J33201) in order to introduce modularity for easy promoter switching. By the end of the bootcamp, we confirmed via Sanger sequencing that both versions of our arsenic reporter had been successfully cloned into DH5a, electrocompotent E. coli cells. The next step would be to get the plasmids into our Shewanella strain lacking mtrB on the chromosome (JG700).
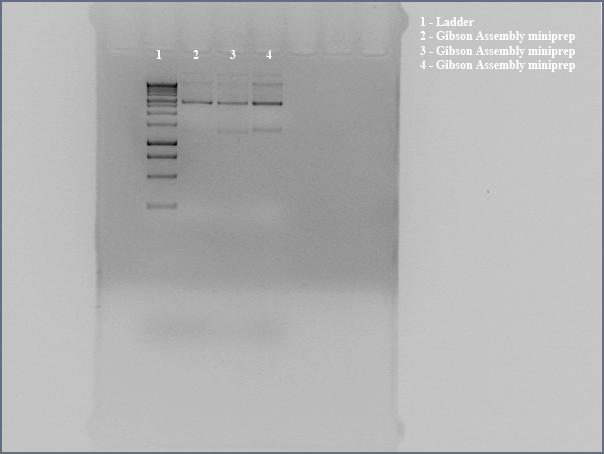
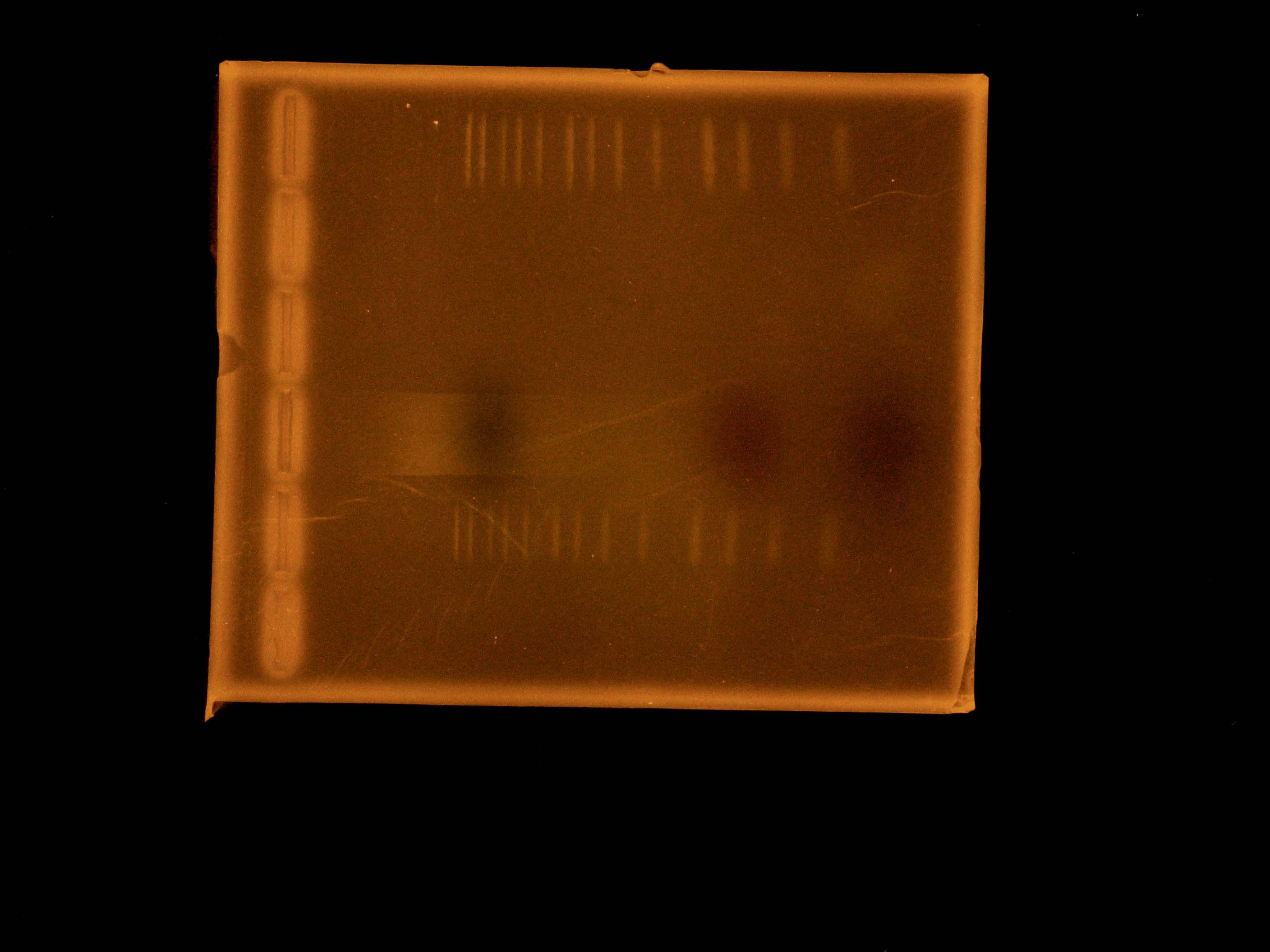
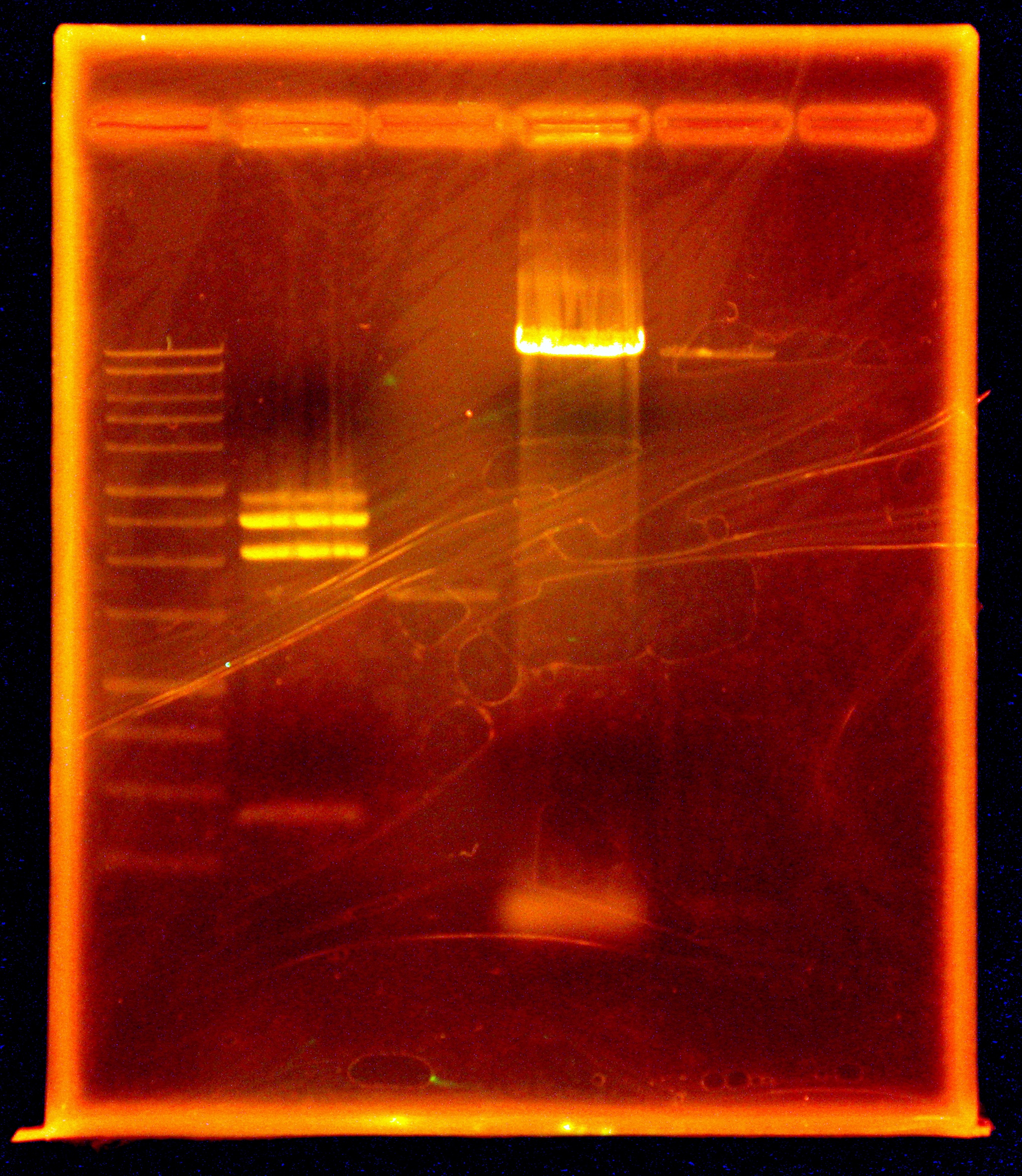
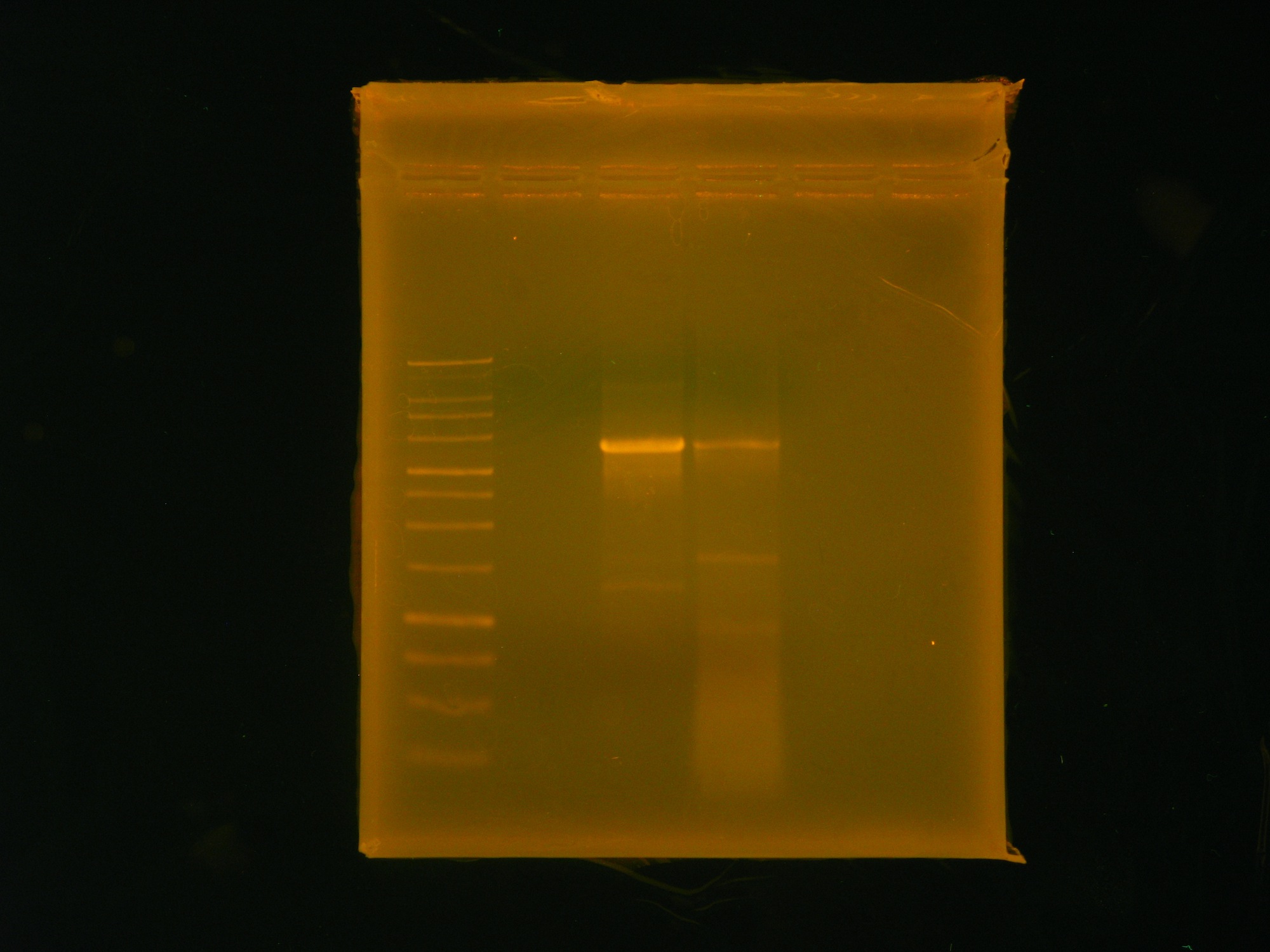
Taylor’s group had one goal: To get internal PstI and NotI cutsites out of our 10kb naphthalene-degrading (nah) operon while constructing the final plasmid to be transformed into Shewanella. By using mutagenic PCR primers that would introduce silent mutations to get rid of the non-BioBrick compatible internal cutsites, the group planned on ripping the nah operon apart, so to speak, and putting it back together again and into a pBMT-1 backbone via Gibson assembly. Using the NAH7 plasmid (containing nah operon) from Pseudomonas putida G7 (kindly provided by Dr. Gene Madsen), Taylor’s group completed all PCR steps necessary for Gibson assembly, incubated the PCR products with Gibson master mix, and transformed the Gibson products into DH5a—yielding three transformants. At the end of bootcamp, the group ran supercoiled plasmid (miniprepped from transformants) on a gel—as an initial screen for successful construction of our naphthalene-degrading plasmid. The gel showed distinct bands for all three lanes, but the screen wasn’t definitive, so the next step was to digest the Gibson products to check for correct band length, and to submit DNA for Sanger sequencing.
June 24th-30th
This week we began our work to construct our salicylate reporter plasmids using a sensing region including the transcriptional activator nahR. We also continued our work with the Gibson mutagenesis of the nah operon (which degrades naphthalene to salicylate) by attempting to confirm successful construction. We also began our adventure in attempting to transform into Shewanella via electroporation.
June 24th, Sunday
June 25th, Monday
June 26th, Tuesday
June 27th, Wednesday
This morning, Steven made glycerol stocks of the 3 Gibson colonies grown overnight. He then did minipreps on the 3 Gibson colonies followed by double digests using PstI and KpnI to check whether the Gibson procedure was successful.
Around noon, Steven and Dylan attempted the Myers and Myers Shewanella Electroporation with p17c (pSB3C5). Dylan and Swati then set up a Phusion PCR to amplify the salicylate-sensing region out of BBa_K228227 (to eventually control the expression of MtrB), before running a gel of the restriction digests of the Gibson products. The gel was determined to be inconclusive in showing that our Gibson colonies appeared to be successful, since our ladder ran very strangely.
That night, Spencer and Steven set up overnight cultures of JG700, nahR, and the 3 Gibson colonies.
June 28th, Thursday
This morning, Dylan and Caleb did minipreps of all the cultures from the day before. They then set up a gel of Phusion PCR of nahR from Wednesday.
That afternoon, Swati set up a transformation of p14 into DH5alpha cells. The cells were recovered for 1 hour at 37C in LB before plating. Dylan set up sequencing of the Gibson products with our newly designed primers while Shweta gel extracted the nahR from the gel set up by Dylan and Caleb. After Shweta’s gel extraction, Swati set up a double digestion of the product with EcoRI and AscI.
That night, Steven ran a gel of the nahR digest while Spencer set up overnight cultures of p14, p16, pSB3C5, and pBBRBB.
June 29th, Friday
In the morning, Dylan set up a Vent PCR to amplify p21 and his previous Phusion PCR band. That afternoon, he gel purified the PCRs and set up a double digestion with EcoRI-HF and AscI. Spencer miniprepped the overnight cultures of PL14-PL20.
Later that night, Dylan set up another Phusion PCR to amplify the nah operon from Gibson colony 1 and to append Biobrick cutsites into the product for future ligation into pSB3C5. Swati started the gel extraction of p21.
June 30th, Saturday
Today Dylan started out the day by running the PCR of the Gibson colony 1 on a gel, gel extracted the band, and set up another Phusion PCR with an extension time of 3 minutes to try and get more of the construct.
That afternoon, Swati came back to finish her p21 gel extraction. She then dephosphorylated pBBRBB+mtrB for ligation with p21. Swati desalted the ligation on Millipore membrane paper and electroporated her ligation into DH5alpha. After letting the cells recover in LB for 1 hours, Swati plated her ligations.
Later that night, Steven and Spencer set up two transformations into PNNL electrocompetent Shewanella one at a voltage of 0.75 kV, and the other at 0.55 kV prescribed by Myers and Myers.
July
July 1st-7th
A lot of troubleshooting occurred this week. Once we figured out that SYBR Green was causing our gels to run strangely, we switched to staining with EtBr after running the gel. This approach gave us better results. We also continued to try electroporation protocols for transforming Shewanella, and continued to work on PCRing out the salicylate-sensing region.
July 1st, Sunday
Dylan and Caleb set up two gels for electrophoresis. Caleb's was 1% agarose in BIO-RAD Mini-Sub Cell system for continuation of ladder test using SYBR Green, containing NEB 100 bp ladder, NEB 2-log ladder, Promega 1 kb ladder and run at 100V. Dylan's was 1% agarose in Owl box using ethidium bromide, containing the nah operon PCR product from previous night, and run at 55 V.
Our plates of DH5a transformed with p21 ligated into pBBRBB with mtrB grew only one colony, possibly contamination. Dylan ran a colony PCR and got a smear, suggesting that the PCR of p21 or the ligation did not work.
Because we learned that our SYBR Green was causing ladder to run strangely, Dylan decided to redo a Vent PCR to amplify the salicylate reporter region out of p21.
Also, a liquid culture of JG700 was prepared, as well as replating of p21, p22, JG700, JG1220, JG1537, JG1219. (See: strain list)
July 2nd, Monday
Today, Dylan and Caleb ran a gel of a Vent PCR of p21 (the PCR product being the salicylate reporter) at 55 V. Additionally, Caleb decided to run a control gel at 100 V to determine whether higher voltage was a factor in our previous ladder problems (in addition to the SYBR Green stock). While we determined that the higher voltage did not cause our ladder issues, we did not see any bands from the p21 PCR (gel image not included).
Dylan prepared electrocompetent cells using the Myers and Myers protocol. Modified protocol using two 5mL cultures @ 4000g for 10 min. Washed with 2 mL sorbitol, resuspended in 100 uL sorbitol. First electroporation ts = 2.32 ms, second ts = 2.02 ms. Added 1 uL of plasmid (575.5 ng) to each cuvette. First used .60 V, then 0.55 V, both with R = 400 ohms.
Mark and Danielle started liquid cultures of S1, S9, S10, S11, S15, S16, S18, S27 (See: strain list)
July 3rd, Tuesday
Caleb miniprepped S16 (p15), S22 (p21), S9 (p8), S10 (p9), S11 (p10), S18 (p17), and S15 (p14). (See: strain list) Instead of a single EB elution, Caleb did two elutions, 30 ul each. The double 30 ul elution turned out to be effective at recovering a usable amount of DNA in the second elution, so we're including it on our miniprep protocol for future minipreps.
The rerun of our Gibson sequencing failed again, so we tried a more roundabout method of determining whether our 3 Gibson transformants have complete nah operons or not. We digested them each with BamHI, expecting specific fragment lengths on a gel electrophoresis.
When Dylan and Mark were attempting to determine what volume of ethidium bromide should be used in the gel, they came to the conclusion that better images may result from staining the gel in dilute ethidium bromide after running the gel, rather than including the stain in the gel. This experimental first staining used 200 mL of water conaining 20 uL of 10 mg/mL ethidium bromide, with gentle agitation for 1 hour. This stained the bands well, but also provided a large amount of background and took a very long time.
We only saw one band on each, all of them at about 3.6kb.
Because no transformations from our previous competent freezer stocks were successful, Dylan decided to make a starter culture of Shewanella strain JG 700 in preparation for making new competent stocks using the PNNL Protocol. Swati and Tina used this starter culture to complete the preparation.
July 4th, Wednesday
In the morning, Dylan, Swati, and Danielle prepared p8, p9, p10, p14, and p15 for sequencing (from the minipreps that Caleb preformed the previous day).
The team took the rest of the day off and had a barbeque at Buttermilk Falls. There was an excess of food, spontaneous song, and fireflies.
July 5th, Thursday
Upon arriving to the lab, Dylan dropped off the sequencing tubes we'd set up on the morning of the 4th for analysis. He also decided to do a Colony PCR of the potential transformant with the salicylate reporter plasmid. However, when setting up this reaction, Dylan realized that we'd used the wrong sequencing primers (we'd used the standard VF2 BioBrick primer instead of a custom primer for the pBBRBB backbone). Consequently, Mark and Dylan resubmitted p14 and p15 for sequencing while the colony PCR was ongoing. After visualizing the PCR products using DNA Gel Electrophoresis, Dylan concluded that the colony we'd screened did not have our plasmid of interest, and was the result of contamination.
Similarly, Dylan noticed small, evenly spaced colonies on all of the JG700 transformant plates from the Myers and Myers procedure we'd undertaken on the 2nd of July. Because none of the colonies looked red (as they should have if they were replicating pSB3C5), Dylan concluded that the chloramphenicol on the plates had degraded, and the colonies were resultant from untransformed cells.
Because the colony PCR of the potential salicylate reporter didn't yield good results, Dylan and Youjin set up another Phusion PCR to amplify the salicylate-sensing region from p21. Simultaneously, Mark made SOB media for recovery after future transformations, as we'd just received the ingredients for the broth.
Because we needed to submit for sequencing twice (because we used the wrong sequencing primers), Swati et al. set up liquid cultures of p21, p8, p9, p10, p17, and p14 in order to have more DNA in the freezer. We like having DNA in the freezer. (See: strain list)
July 6th, Friday
Caleb miniprepped p21, p8, p9, p10, p17 from the liquid cultures set up the previous night. (See: strain list) We couldn't miniprep p14, since – after checking notes from the previous night – we learned that the culture was made with the wrong antibiotic (chloramphenicol instead of kanamycin).
Some sequencing results came back, however the Gibson results are still confusing.
Also, a gel was run for the p21 PCR product.
July 7th, Saturday
July 8th-14th
The focus of this week was to transfer our arsenic sensing plasmids to Shewanella strain JG700 via congugation with E. coli strain WM3064. After struggling to electroporate control plasmids, we consulted with Dr. Gralnick who reccomended proceeding with conjugation and kindly provided E. coli strain WM3064. This strain is auxotrophic for 2,6-Diaminopimelic Acid (DAP), which makes the strain useful for selecting against non-Shewanella conjugants.
Another major decision this week was to give up on Gibson assembly - our sequencing results suggested that strange things had happened, and since we already had PCRed the full nah operon out of P. putida, we decided proceeding with Gibson was not an efficient use of time. We were also unsure as to why p21's PCR was repeatedly failing, and decided to submit the part from the registry for sequencing, to try to troubleshoot what was going wrong.
July 8th, Sunday
July 9th, Monday
Dylan performed a Phusion PCR with a single annealing temperature of 59 degrees Celsius using primers 27 and 28 for the final time. In the future, new primers will be used. The purpose of this PCR was to amplify p21 (See: strain list). The PCR product was analyzed using agarose gel electrophoresis to determine if the desired 1.127 kb fragment is present. We talked to Dr. Jeff Gralnick about electroporating Shewanella, and his recommendation was to try conjugation instead. To transform our plasmids into Shewanella with conjugation, we had asked Dr. Jeff Gralnick for WM3064 E. coli for which plates containing 2,6-Diaminopimelic Acid (DAP) are required. In preparation to receive this strain, the plates with DAP and DAP/kanamycin were prepared.
July 10th, Tuesday
Caleb miniprepped strains containing Gibson products and the arsenic promotors without BamHI cutsites: plasmids p8, p9, p10, p15, and p16 (See: strain list) Also, our new freezer arrived. We cleaned and welcomed our new friend.The WM3064 came in and we plated from the agar stab. Dylan set up two 18 mL/hr continuous flow reactors using 10x diluted LB and innoculated one of the reactors with JG700 and one with wild type Shewanella oneidensis MR-1.
July 11th, Wednesday
Dylan started a WM3064 subculture in the morning before meeting Swati, Shweta, Caleb, and Danielle at the Boyce Thompson Institute for Plant Research to give a presentation both on our project and on the broader applications of synthetic biology to the energy industry to a group of high school teachers from New York state. After the presentation, Dylan and Danielle prepared plates containing DAP/chloramphenicol and DAP/ampicillin to grow transformed WM3064 on, while Mark continued preparing electrocompetent stocks of WM3064 with the subculture that Dylan had started.
We transformed things later that day.
July 12th, Thursday
Shweta prepared a glycerol stock of WM3064 for future use, and Dylan plated some remaining transformed WM3064 hoping to get more colonies of the successfully transformed cells.
Dylan and Mark submitted p8, p9, and p10 for sequencing using two different primers for each plasmid.
The previous night's p21 PCR seemed to have failed, even with the new primers. Based on the gel run by Claire, then analyzed by Dylan and Caleb.
July 13th, Friday
Today began with Dylan and Caleb setting up conjugations between our transformed donor E. coli and S. oneidensis.
The sequencing results for p8, p9 and p10 all came back as failed, so it was concluded that the Gibson assembly failed. There were five locations on the nah operon at which sequencing could begin, of which we considered all but the fourth. Sequencing at the first location resulted in a sequence that corresponded to the fifth sequencing location, with some sequence before it and all of the expected sequence after it. The fifth sequencing location produced the expected sequence. Then the middle two regions which we considered all resulted in failed sequencing. This has led us to the conclusion that segments that are required in the operon are missing, and thus the assembly failed. Dylan and Caleb began attempting to look into the possibility of this being a cause, and some potential solutions.
In discussing why p21 PCR seems to continue failing, the idea of having p21 sequenced arose. Dylan prepared the sample and submitted it for sequencing. Meanwhile, Mark set up yet another Phusion PCR of p21, this time with a higher annealing temperature of 61 degrees Celsius and a decreased extension time of 18 seconds. The goal was to prevent the mispriming which seemed to have occurred in the previous day's attempt.
Later that day, Dylan set up yet another PCR with a decreased annealing temperature of 55 degrees Celsius in order to empirically narrow-in on the optimal annealing temperature for the amplification of the salicylate sensing region from p21.
July 14th, Saturday
Swati ran a gel of 5uL of the p21 PCR that Mark set up yesterday. After staining, the DNA ladder was well visualized but there were no bands in the p21 lane, suggesting that the PCR did not work. In a fit of rage, Swati yelled at Maneesh for going to get a drink of water - yet another innocent victim of failed PCR. Then, after a full team meeting, we all came together for a dumpling party. Noodles, dumplings, and gnocchi of all kinds were eaten by the voracious team. In the spirit of true, unwavering scientific inquiry, we conducted a novel experiment whilst feasting on these delectable treats. The result: fried Dorito dumplings are delicious.
July 15th-21st
For this week, we began to conjugate our constructs into Shewanella and Dylan began seeding our reactors with WT Shewanella and with p14 to begin collecting data. We’ve also started work with the Anderson series of constitutive promoters for future work in anticipation of constructing control plasmids with constitutively produced MtrB.
July 15th, Sunday
Dylan noticed that we had colonies on the plates re-streaked from the original plates of conjugated S. oneidensis. He started overnight cultures of S20 (See: strain list) in kanamycin so that we can sequence and make glycerol stocks. He also started overnight cultures of w.t. S. oneidensis to inoculate into new reactors in Riley Robb, which will be used as positive controls.
July 16th, Monday
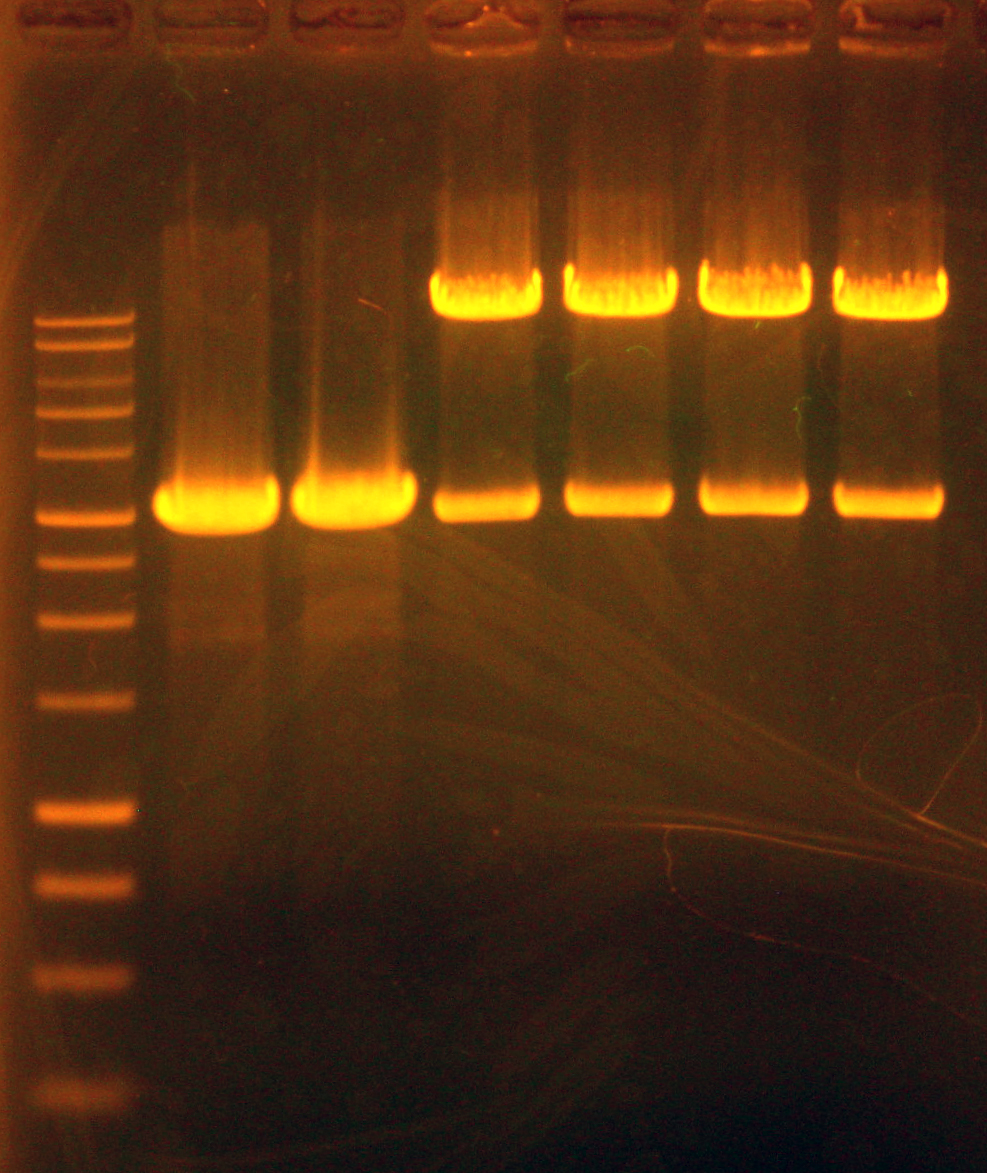
Dylan ran another Phusion PCR of the entire nah operon, since we got a lot of mispriming the first time we ran it; we believe this was because we had used the optimal annealing temperature for Vent Polymerase (55C) with Phusion Polymerase. Dylan set up the new PCR correctly, with an annealing temperature of 66C and a lengthened final extension time of 15 minutes to account for the size of the nah operon (~10kb). Caleb ran a gel of the PCR and visualized a single band ~9.5kb. PCR of the nah operon out of p20, P. putida (See: strain list) was successful! Tina and Swati gel extracted and quantified, extracting two samples at 38.8 ng/uL and 27.2 ng/uL.
Spencer miniprepped p14, our arsenic reporter part (arsR + mtrB w/ BamHI cutsite), from S20 (See: strain list) for sequencing, recording yields of 44.3ng/uL & 35.8ng/uL (colony 1) and 50.6ng/uL & 53.5ng/uL (colony 2) from quantification. Dylan and Tina set up transformations of p15 and p16 (See: strain list), the arsenic reporter parts without a BamHI cutsite, so conjugations with S. oneidensis can be done in the next few days. They also set up a transformation of the miniprepped BBa_J01003 with the oriT mobility gene. Because of our lack of success with electroporation, we are planning on trying conjugation of our constructs into S. oneidensis. However, this requires that all our plasmids have the mobility gene. pSB3C5, the plasmid we are going to use for the nah operon has no mobility gene. Thus, we are going to try two things: to clone oriT from iGEM kit plates into pSB3C5, and to amplify the mobility gene out of one of our own plasmids and clone that into pSB3C5.
July 17th, Tuesday
Shweta set up a Phusion PCR of p21 (nahR and Psal) to try and extract the salicylate-sensitive promoter again. Sequencing of p21 showed a stem loop sitting upstream of the ENX biobrick cutsites, which was not expected and likely the reason for our unsuccessful PCRs. Before redesigning primers, we are going to try PCR with the standard iGEM forward sequencing primer and the same reverse primer that we designed for p21.
July 18th, Wednesday
Dylan set up p15k, p16k conjugation plates from overnight cultures of transformed WM3064 and JG700. Dylan let these plates incubate for 8 hours, and then streaked for single colonies on kan plates with E.coli and Shewanella controls. Caleb miniprepped p24a (BioBrick part with oriT) from overnight cultures. He then desalted an overnight ligation of the nah operon in pSB1C3 and transformed into DH5a. Dylan also trekked over to Riley Robb to set up a reactor for inoculation tomorrow. That night, Spencer set up overnight cultures of p25a, p28a, and p31c.
July 19th, Thursday
Only observed 2 possible colonies from transformants of yesterday's ligation (nah operon in pSB1C3). We restreaked from one of these colonies along with a DH5a control.
We got single colonies on the JG700+p15 and JG700+p16 plates, and no growth on control plates. Thus, our tentative/optimistic conclusion is that the conjugation was successful. Dylan and Caleb made reference plates of the picked colonies, and grew up liquid cultures from same colonies for sequencing.
Dylan and Caleb then miniprepped p25a, p28a, p31c from overnight cultures set up by Spencer and made glycerol stocks of S25, S28, and S31. That afternoon, Dylan also inoculated a reactor in Riley Robb with S20, our engineered arsenic reporter strain.
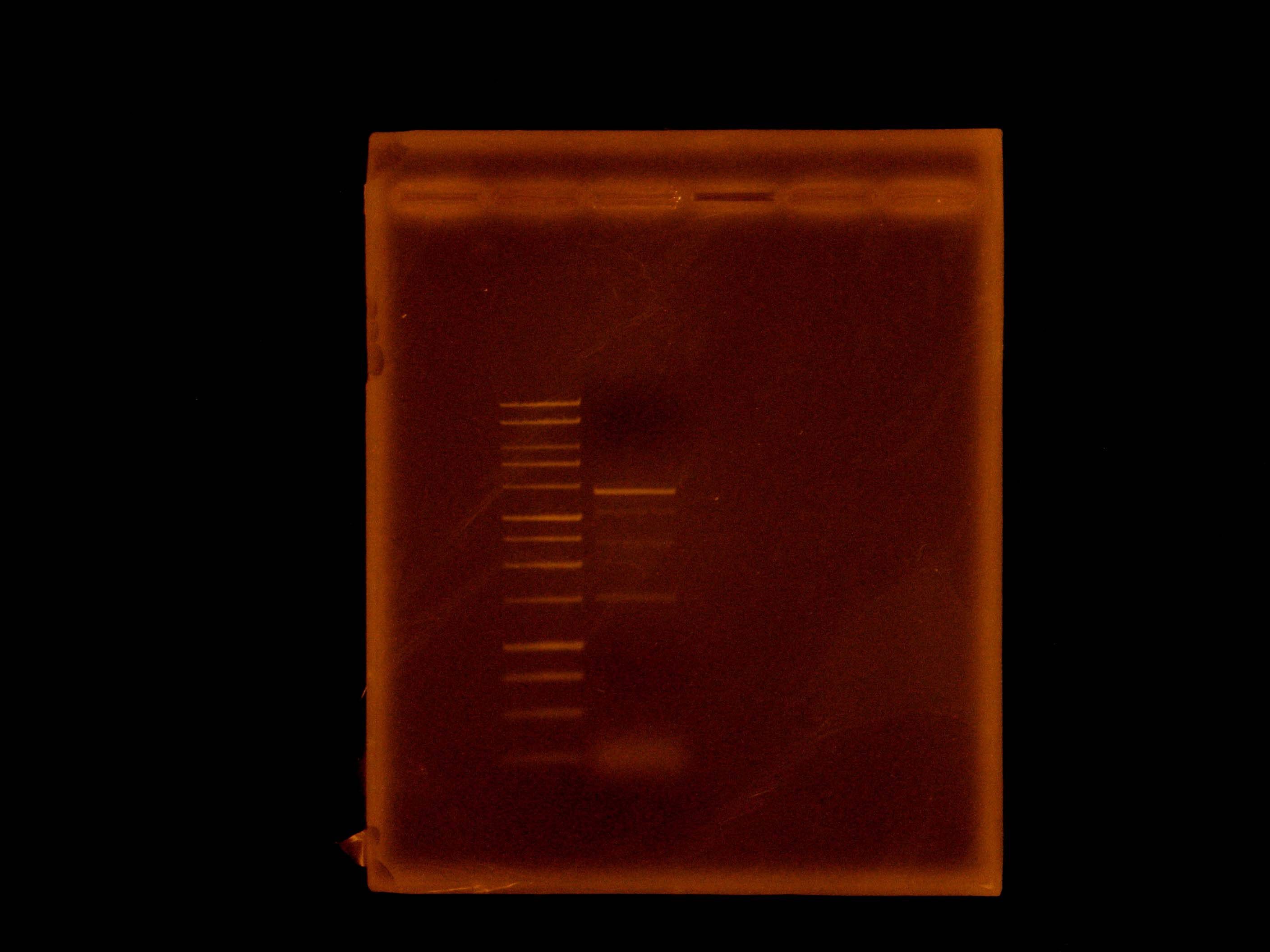
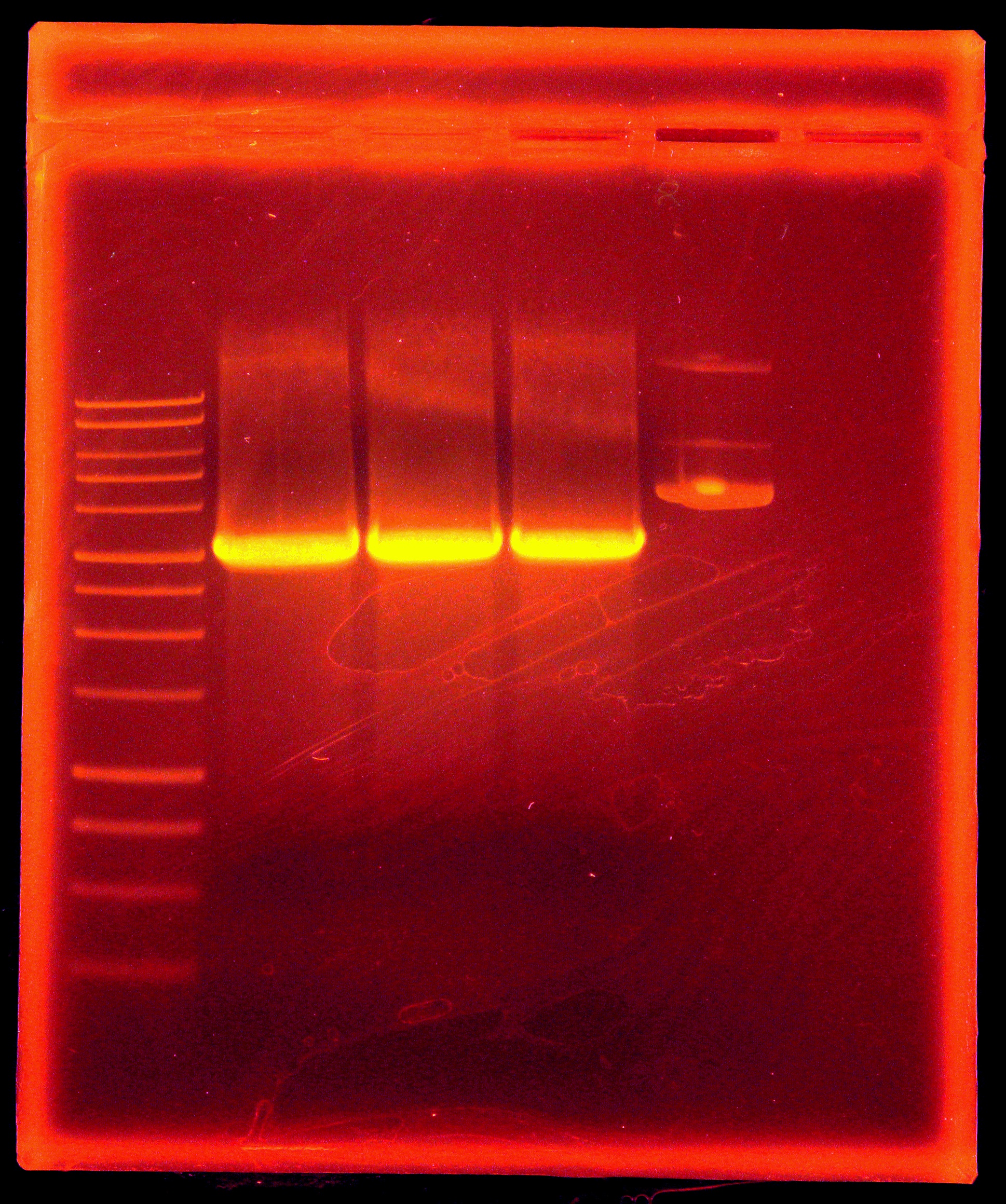
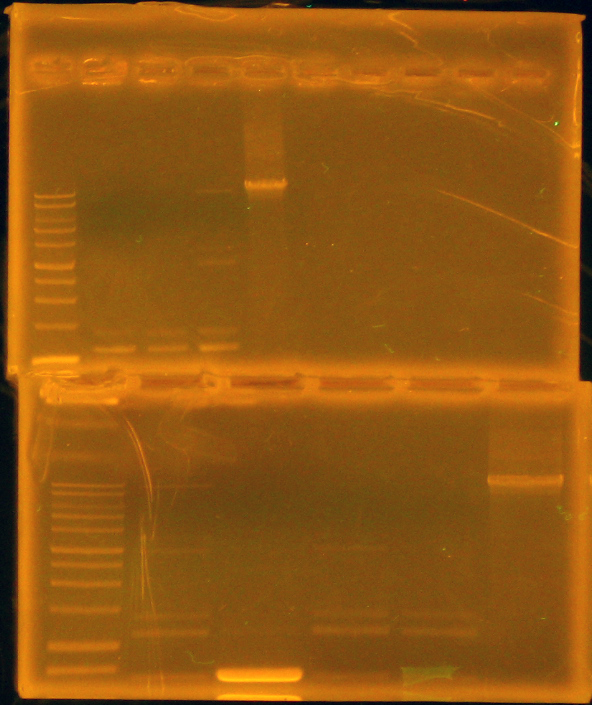
We then set up a digestion of the p21 PCR product along with our miniprepped p14 with EcoRI and AscI. We purified the p21 PCR digestion with Omega Bio-Tek E.Z.N.A. MicroElute DNA Clean-Up Kit. We dephosphorylated p14 digestion with Antarctic Phosphatase, and ran the entire mixture on a gel. (Picture of gel). That night, we gel extracted the 6kb band with our Qiagen gel extraction kit and set up overnight cultures of a p26a, 027a, p29a, p30a (our Anderson series constitutive promoters), and p14k.
July 20th, Friday
This morning Shweta and Tina miniprepped p26a, p27a, p29a, p30a (Anderson series constitutive promoters), and p14k from overnight cultures. Dylan set up a 30 minute room temperature ligation to connect the salicylate reporter with digested p21 PCR product and p14 (isolated yesterday, quantified today). Because Shewanella grows more slowly than E.coli, Claire did a second miniprep of p15k and p16k from JG700 in order to submit the plasmid for sequencing to confirm that conjugation was successful. In anticipation of the failure of Dylan's ligation, Swati set up 3 Phusion PCR reactions in parallel, each with the same template (p21), and repeated the parameters that had proven successful (no mispriming) previously. Dylan also set up overnight cultures of the strain with the nah operon in pSB1C3, S18 (DH5a + pSB3C5/p17c), and WM3064+p14.
July 21st, Saturday
July 22nd-28th
The goal of this week was to transform DH5α with nah operon (p20 PCR + p25a, p27a, or p29a).
July 22nd, Sunday
Danielle and Dylan digested p14(Arsenic reporter) and p21(Salicylate sensing region) for ligation. Swati ran a Phusion PCR to amplify p21. Then Dylan ran a gel electrophoresis and did gel extraction to isolate p21 PCR digest and p14 backbone.
July 23rd, Monday
Dylan quantified the gel extractions, quite successfully with a newly invented protocol. Then he dephosphorylated p14, and submitted some DNA for sequencing. At one point, the Magical Graduated Cylinder of Elmira (500 mL) fell from the sky and shattered in the sink. Some voodoo was clearly in the air. It appears that the ligation products from the day before were either not created or not successfully transformed, as the plates contained no colonies.
Caleb and Tina prepared more kanamycin and chloramphenicol plates while Mark desalted Dylan's ligation product. Then Dylan, Mark, and Tina transformed DH5α with the ligation product, p33k, p14k, and p31c (See: strain list).
July 24th, Tuesday
In the morning, Dylan did Phusion PCR of p20(nah operon). Then Caleb and Dylan did a double digestion of p20 PCR and p17c for ligation. Dylan ran a DNA gel electrophoresis to confirm that the previous PCR was successful. Steven performed gel extraction to isolate p17c backbone fragment.
July 25th, Wednesday
Mark set up two ligations of a p20 PCR product and p17c, one at standard concentrations and one at very high concentrations. (See: strain list) p20 PCR was cut at Xbio1 and Spe1, which have compatible sticky ends. This means that p20 PCR can be ligated into backbone in two directions, only one of which is useful. These were desalted and transformed by Mark and Dylan, along with p33k. Meanwhile, Tina and Chie set up double digestions of p24a, p20 PCR product, and single digestions of p25a, p27a, and p29a.
Dylan ran two gels of the double and single digestions. The single digestions of the Anderson series promoters showed single bands, which ran slightly faster than the supercoiled DNA control. This may be because the supercoiled DNA was nicked. The gel of the oriT double digestion showed more long bands than expected – possibly a result of star activity – but had a band ~400bp which was interpreted to be the insert of interest.
July 26th, Thursday
Caleb gel purified the digestion products of p24a, p20 PCR, p25a, p27a, and p29a. Danielle dephosphorylated products of p25a, p27a and p29a in preparation for ligation.
July 27th, Friday
In the morning, Claire miniprepped p11k, p17c, and p33k from the overnight cultures that Dylan had set up. Meanwhile, Dylan heat killed the ligase from the overnight ligations(p25a, p27a and p29a), while Caleb prepared to desalt the ligation mixtures before electroporation via drop dialysis. Shortly thereafter, Dylan and Caleb electroporated the four desalted mixtures into DH5α, allowing the cells to recover one hour in SOC before plating. If these transformations are successful, we will have cells that carry plasmids expressing the nah operon under the control of three different constitutive promoters with varying strength, as well as a cell line that carries a plasmid that confers chloramphenicol resistance, has a p15a origin of replication, and an origin of transfer. Eventually, the nah operon will be ligated as an insert into such a backbone.
In the evening, Dylan and Tina started 30 mL overnight cultures of S24, S25, S27, S29, S15, and S17 to miniprep from the next morning. S24 carries a plasmid with an oriT, which will be miniprepped in case our previous ligation of an oriT into p17c failed. S25,27, and 29 all carry plasmids with Anderson series constitutive promoters of varying strength, which will be used to set up fluorescent controls to monitor mtrB expression levels. S15 and S17 carry our engineered arsenic reporter plasmids, which will be further modified to facilitate control studies in mtrB expression.
After setting up the cultures, Dylan and Swati left Weill and headed over to Riley Robb where they assembled three new reactor setups in the Angenent lab. Two of these reactors will be run in continuous flow operation with a BioLogic potentiostat, while the other will be run in batch mode with a CH Instruments potentiostat. Tomorrow, one continuous flow and one batch reactor will be inoculated with wildtype Shewanella oneidensis MR-1, while the remaining continuous flow reactor will be inoculated with our engineered arsenic reporter strain, S20. The purpose of running the continuous flow reactors is to better define the maximum current output we can expect from our induced strains in response to analyte, and to repeat an experiment carried out on the CH potentiostat for basal activity from the uninduced arsenic reporter. Because we were unsure of the veracity of the data from our previous experiment using the CH potentiostat, we are running the batch reactor to see if the observed current response is what we'd expect from wildtype Shewanella.
July 28th, Saturday
Today, Swati did six minipreps in the morning and it took her four hours...and it paid off! The nanodrop told us that our highest yield was 812.6 ng/uL.
While Swati was devoting her soul to the E.Z.N.A kit, Dylan continued to set up reactors in the Angenent Lab, making reference electrodes for the three reactors, and getting the pumps set up for the continuous flow reactors. After our weekly team meeting, he inoculated the reactor.
Swati prepared overnight cultures and reference plates, while Danielle prepared kanamycin plates and did autoclave.
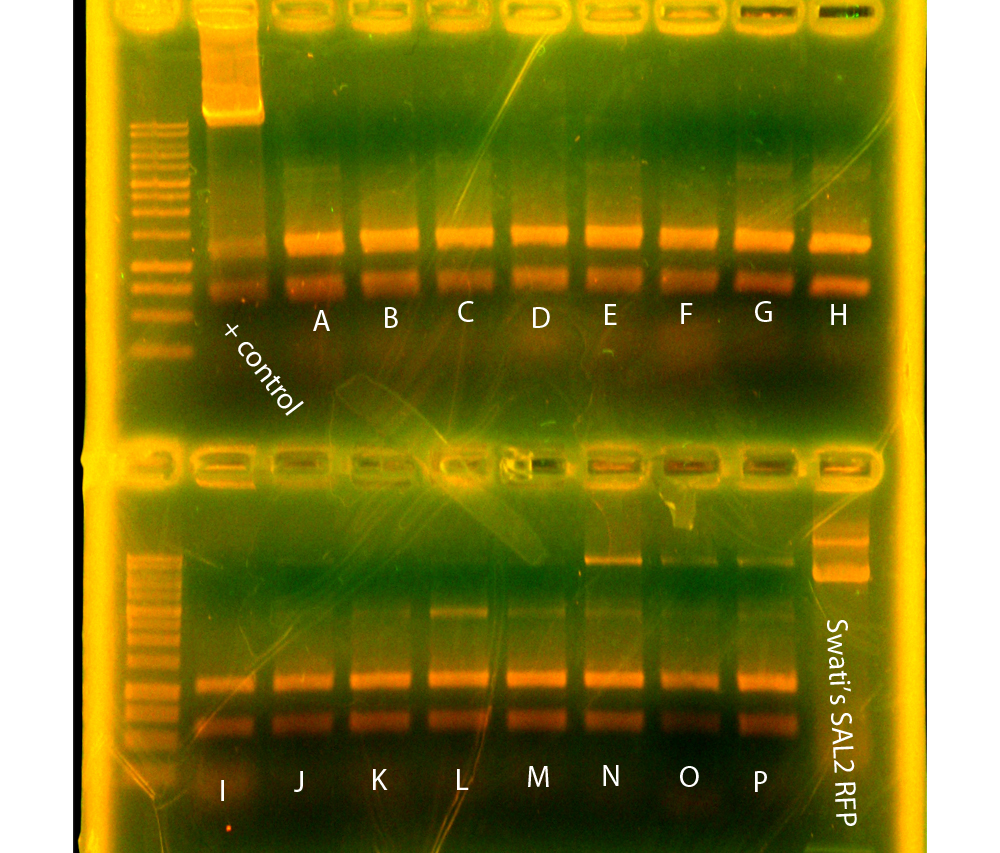
In the evening, Dylan and Swati did digestions for RFP controls and SAL2 reporter. Also, SAL reporter was transformed into WM3064.
July 29th-31st
SAL (the version of the salicylate reporter with the BamHI cutsite) was conjugated into JG700, and we created SAL2 (salicylate reporter without BamHI cutsite). We prepared constitutive controls and our reporters for fluorescence testing. We also continued cloning work to get the nah operon with a constitutive promoter ready to conjugate into JG700.
July 29th, Sunday
Apparently, Swati has yet to get over her miniprep craze. While most of the wetlab hiked through Danby State Forest, Swati did more minipreps: p25a, oriT + PSB3C5, and nah operon + p29a. The cultures of S25 and nah p29 were bright magenta, while oriT in PSB3C5 was a pale tan. However, the last of these contained an unidentified crystalline substance, possibly a byproduct of producing the nah operon. Two other cell cultures also didn't grow, which was disheartening, as it meant there were less minipreps for Swati to do. Interspersed between the copious miniprepping were two gels to purify the digestions from the previous evening, all of which appeared to work! Dylan and Claire, returning from their nature trek, joined Swati in lab, finding Shweta and Eric along the way. While Dylan took over Swati's gel extraction, Swati, Shweta, and Claire discussed upcoming outreach events. As Ecologist Claire explained the feeding habits of Tomiculus troutus (AKA Tommy the trout), Eric began to feel left out and, in the process of showing off the website, discovered Dylan's odd love for typography. After spending roughly an hour accomplishing nothing, Dylan and Swati set up liquid cultures in preparation to put SAL into JG700. Dylan returned to his reactor-ridden lair for a short spell.
July 30th, Monday
In the morning, Dylan set up conjugations between WM3064+SAL and JG700 while Caleb quantified the minipreps and gel extractions from Sunday. After quantification, he submitted nah+p29a and oriT+p17c for sequencing. Dylan and Mark dephosphorylated the digestions of SAL with SpeI & PstI, p14k with SpeI & PstI, p16k white SpeI & PstI, p16k with EcoRI & AscI, and p11k with EcoRI & PstI. Tina and Danielle then set up ligations of mRFP+SAL, mRFP+p14, and mRFP+p16 to put mRFP downstream of the arsenic and salicylate reporters, in order to be able to characterize our induced promoter strengths by measuring fluorescence. We also ligated p16+SAL to make SAL2, the salicylate reporter without the BamHI cutsite. Finally, we ligated p25, p27, and p29 (the Anderson promoters with mRFP) into pBBRBB, in order to be able to put them into Shewanella.
July 31st, Tuesday
Mark made DH5α electrocompetent stocks. Dylan checked conjugation plates to put SAL into JG700. He then made overnight cultures of JG700+SAL and S31 (PSB1C3, as well as WM3064 to prepare competent stocks. Dylan autoclaved a reactor in Riley-Robb and set it up in batch with M4 media, and inoculated with wild-type S. oneidensis MR-1. The purpose was to repeat a previous batch experiment in order to be sure that it is something about feeding with LB that is decreasing current production.
Sequencing results came back on nah+p29 and oriT+p17c and things looked good. Consequently, Dylan decided to digest nah+p29 with EcoRI and SpeI, and oriT+p17c with EcoRI and XbaI in order to eventually ligate the nah operon with a constitutive promoter into the p17c backbone, upstream from the oriT sequence (in order to allow the plasmid to be conjugated into Shewanella). Claire prepared these digestions, along with digestions of p31c and p20 PCR product (in order to get the nah operon into pSB1C3).
Dylan and Caleb desalted the previous night's ligations and than transformed into WM3064, since we will want to get plasmid into Shewanella.
August
August 1st-4th
August 1st, Wednesday
In the morning, Dylan noted that 5 of the 7 transformations from Tuesday produced plates with colonies. Those that worked were three Anderson series constitutive promoters (with downstream mRFP) in pBBRBB and the versions of our two arsenic reporters with mRFP downstream of mtrB. In the evening, Swati and Dylan prepared overnight cultures from all transformants and JG700, both to set up conjugations to get plasmid into Shewanella and to screen isolated plasmids for successful ligation. We will use these constructs to characterize the activity of the arsenic-sensitive promoter in Shewanella with respect to known constitutive promoter strengths. The two transformations that failed were versions of the salicylate reporter.
After staring at plates, Dylan ran the nah+p29, oriT+p17c, p31c, and nah operon (from p20) digestions on a gel, extracted the fragments of interest, and then dephosphorylated the backbone. The isolated digestion products were used later used by Steven, who set up two overnight ligations – one to put the nah operon, with a constitutive promoter, on a pSB3C5 backbone with an added oriT sequence, and one to put the nah operon into the MCS of pSB1C3 for subsequent site directed mutagenesis.
While Dylan ran his gel, Caleb miniprepped from the JG700+SAL and S31 cultures. After quantification, SAL plasmid isolated from JG700 was submitted for sequencing in order to confirm successful conjugation.
Meanwhile, Mark decided that he enjoyed making DH5a electrocompetent stocks on Tuesday so much that he had to make more WM3064 electrocompetent stocks today. Starting from subcultures that Dylan had prepared, he prepared the stocks, and was assisted by Danielle.
August 2nd, Thursday
Caleb and Dylan created conjugation plates of p14RFP, p16RFP, and p25-29BB as well as miniprepped from the same plasmids in WM3064 (see: strain list). Dylan, Mark, and Tina then desalted and transformed SAL2 and SALRFP into WM3064 as well as p31c_nah and oriT_nah_p17c into DH5a. Tina and Dylan then quantified the minipreps from earlier in the day. Dylan also digested pBBRBB and p33k with EcoRI & PstI to put lac inducible mtrB into the pBBRBB backbone.
August 3rd, Friday
After running a digestion of p33k, the lac inducible mtrB part from the parts registry, we discovered that the part does not include mtrB! We should have seen a ~3.6kb band on our gel after digesting with EcoRI and PstI, but instead isolated a band ~1.5kb. After checking the sequencing on the parts registry we discovered this was because the part didn't include mtrB, so we won't be able to use it as a positive control for inducible mtrB.
Sequencing of p37k-p41k from WM3064 came back good, meaning we can put these control parts, with RFP downstream of our various reporters, into Shewanella via conjugation. Hence, conjugation was carried out.
August 4th, Saturday
This week, it was confirmed that RFP was successfully inserted downstream of mtrB in our arsenic reporter in Shewenella.
Site-directed mutagenesis of the nah operon failed. We continued trying to insert the nah operon into the mobile backbone (OriT) to prepare for conjugation into Shewanella.
August 5th-11th
It was confirmed that RFP was successfully inserted downstream of mtrB in our arsenic reporter parts in Shewanella. Site-directed mutagenesis of the nah operon failed. We are still trying to insert the nah operon into the mobility backbone (OriT) to prepare for conjugation into Shewanella later on.
August 5th, Sunday
Swati yet again was saddled with a massive number of minipreps, since her magic fingers are able to cast yield-increasing spells on minipreps. She miniprepped: SAL2 from WM3064, p37-41k from JG700 (one arsenic reporter with cut sites flanking the RBS, another arsenic reporter without the cut sites, and three different Anderson series promoters with mRFP1 downstream on a pBBRBB backbone), oriT p29nah_p17c from DH5a, and nah_p31c from DH5a. And indeed her yields were impressive, massive, gargantuan! Good job Sorceress Swati.
We will sequence SAL2 and oriT p29nah_p17c to see if we should continue to conjugation, and sequence nah_p31c to see if we can start site-directed mutagenesis to get a biobrick-compatible nah operon on the biobrick backbone. We will sequence or PCR to confirm p37k-p41k's conjugation into Shewanella.
August 6th, Monday
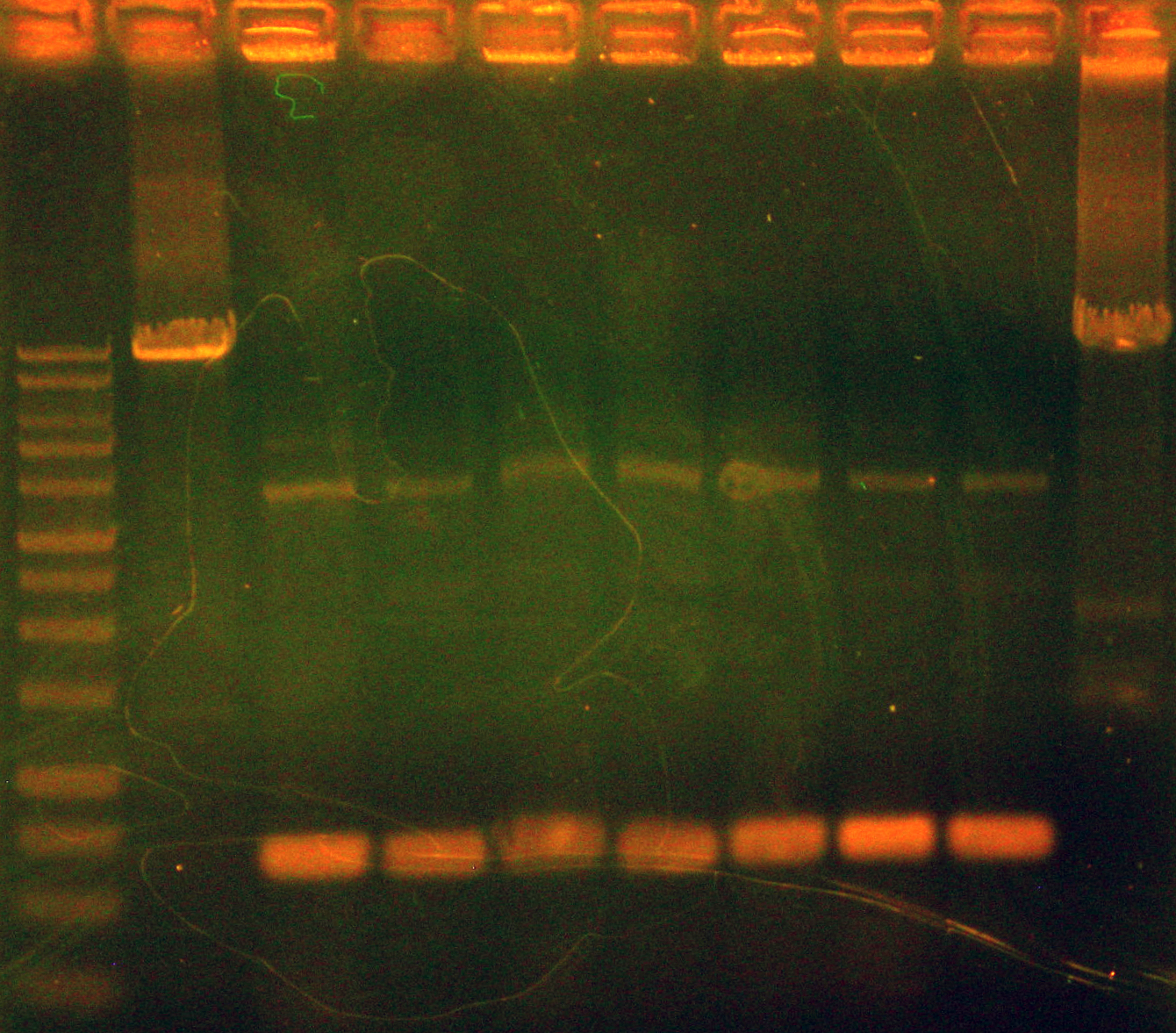
Caleb and Dylan amplified the two different arsenic promoters (p37k and p38k) and ran them on a gel alongside p14 and p16 (the arsenic reporters without mRFP1 downstream) to confirm successful insertion of RFP downstream of mtrB in our arsenic reporter parts in Shewenella. Band lengths appeared in expected places. Dylan is worried that we will not be able to use PCR to definitely confirm that p39-41k worked, so we submitted these for sequencing. Unfortunately, sequencing failed, perhaps because random gunk from Shewanella added noise to the process.
Tina did a PCR cleanup of the nah operon PCR. Dylan also ran a gel of nah operon PCRs to make sure that there was no mispriming. The gel looked good, so he submitted the PCRs for sequencing.
August 7th, Tuesday
JG1531 overnight culture didn't grow. We suspect that the plate Dylan picked from is dead. We'll have to go back to the glycerol stocks if we want to play with mtrE. Dylan set up a continuous flow M4 reactor in morning. Caleb started a liquid culture of MR-1 with which to inoculate the reactor. Checked sequencing results of nah stuff. oriT thing was bad. nah_p31c was good.
Caleb and Dylan proceeded with site directed mutagenesis of nah_p31c, attempting to get rid of the first PstI cutsite in the nah operon. After digestion with DpnI, we purified using the E.Z.N.A. MicroElute kit and quantified. DNA was split into three directions: First, Danielle and Dylan set up another mutagenic PCR using the digestion product as template to get rid of the second internal cut site (we expect lower mutation efficiency because template DNA is not methylated). Second, we transformed DH5a with the mutated plasmid. Third, Danielle and Chie digested both the purified – and hopefully mutated – plasmid and un-mutated plasmid with PstI. Dylan ran these digestions on a gel, along with supercoiled plasmid as a control. Unfortunately, the (hopefully) mutated plasmid never showed up on the gel.
In the evening, we didn't observe growth in the MR-1 culture, so Dylan set up another culture just in case Shewanella was dead and not lazy.
August 8th, Wednesday
Caleb and Dylan performed another digest to check if the second site-directed mutagenesis (that was supposed to get rid of the second PstI internal cut site within the nah operon) worked. Unfortunately, there wasn't enough DNA to see anything when visualized on a gel. They decided to proceed with electroporation into DH5a just in case - however, to use Caleb's terminology, cells exploded - there were probably too many salts in the solution, which causing arcing and a PBBHTTTZZZ! of cells all over the cuvette.
Dylan re-digested p29 nah and oriT p17c to redo a ligation that would allow for conjugation into Shewanella later on. Dylan also re-digested p27 (Anderson series promoter with RFP downstream) and SAL to redo a ligation to create a plasmid with SAL-RFP.
Dylan also submitted several samples for sequencing to make sure that three Anderson promoters with RFP downstream in JG700 and SAL2 (the salicylate reporter without the BAMHI cut site) in WN3064 and JG700 were in the correct sequence.
August 9th, Thursday
Today Dylan chilled with his mom. Well, he tried to. :) Despite the eternal bonds that bind mother and son, plus the 3 billion miles she flew to see him, he still couldn't resist coming in to lab to open packages! And then got sucked into a long discussion of what had to be done for the rest of the day - scientific endeavors taking him away yet again from filial duties. Swati and Claire bonded over dry ice, and Caleb regaled us with tales of dry ice bombs gone awry.
Swati and Caleb miniprepped the first mutagenesis of nah_p31c that had been transformed into DH5a yesterday. They then digested it with PstI-HF to see if the mutation was successful. Unfortunately the mutagenesis failed! We will start over from nah_p31c and lower the annealing temperature at 60degC. The Stratagene kit, which uses PFU ultra, asks for 60degC, but because we are using our own boot-leg protocol with Phusion we did the first try at 65degC, as Phusion usually calls for a higher annealing temperature than the theoretically calculated value. For this second try we will stick to the 60degC suggested by Stratagene and see if we get better results. Also, called NEB to find out if after PCR with Phusion, DpnI will still have activity in the following digestion step in Buffer 4, or if we need to clean up the PCR before digesting with DpnI - they said PCR clean up isn't needed.
We also ran digestions for making the nah operon on a backbone with a mobility gene and the salicylate reporter (w/ BamHI cutsite) with RFP downstream. We cut our mobile backbone, OriT in p17c (pSB3C5), with EcoRI and XbaI, while cutting the nah operon (p29nah) with EcoRI and SpeI. The nah operon with mobility gene must be constructed so that we may conjugate into Shewy and start testing our salicylate reporter. The salicylate reporter and p27a (from the Anderson series), with RFP, were cut with SpeI and PstI. The salicylate/RFP part will be used for troubleshooting the salicylate reporter. Digestion products were run on a gel, extracted, and quantified. Swati then dephosphorylated backbones and ligated both parts.
Sequencing for p39-41k didn't look good, and neither did the salicylate reporter w/BamHI cutsite miniprepped from JG700. The sequencing for salicylate reporter w/ BamHI in WM3064, however, looked good, suggesting that conjugation may not have been as efficient as we had hoped. After a pow-wow we decided not to sequence more colonies, as we are hoping some may be good, and more importantly that if we use qPCR to get quantitative characterization data, we won't need to use the RFP parts. The plates will stay in the fridge as a back-up plan.
In other news: Claire cried because it was difficult to update the notebook with a week's worth of work. Mark's dedication to the notebook is laudable and impressive. Good job team for doing so much! My head can't even comprehend the magnitude of your endeavors.
August 10th, Friday
Caleb miniprepped a plasmid with mtrE from JG1531, and just for kicks, miniprepped from the "exploded cells" from August 8th. Suprisingly, he ended up getting decent yields for both, showing that the electroporation worked despite arcing. Caleb then digested the nah operon of the miniprep with PSTI and NotI to check if the mutagenesis was a success. It was run on a gel alongside a PCR of the Anderson series promoters with RFP downstream and SAL2 from Shewanella (to check if SAL2 and the promoters were sucessfully conjugated after sequencing on Monday failed). Unfortunately, bands did not appear where we expected them to.
Steven and Spencer performed a PCR to get mtrE out of the Gralnick (JG700) plasmid.
August 11th, Saturday
Spencer and Steven checked the nah operon PstI and NotI digest (because yesterday's gel ran weirdly) and their mtrE PCR from the previous day on a gel. Unfortunately, bands did not appear where we expected them to.
August 12th-18th
This week, we:
1)Performed the neccessary work for getting our reporters into the pSB1C3 backbone required for submission. This included the neccessary digestions of our reporters out and ligations into the submission plasmid. Confirmation of successful transformation and ligation will be performed in the following weeks.
2)Confirmed successful transformation of Shewanella with our arsenic and salicylate reporters via colony PCR.
3)Performed the neccessary digestions and ligations to have mRFP downstream of our reporter system so as to do an additional fluorescent testing of the reporters to serve a second form of confirmation of increased transcription in the presence of our toxins.
August 12th, Sunday
Weekend overview: It looks like the mtrE primers are actually working to amplify the part out of JG1531. We'll be putting mtrE in p31c, both so that we may submit the novel part to the registry, and to begin site-directed mutagenesis.
We'll use mtrE mutagenesis as something of a control for nah operon mutagenesis (to see if the large size of the plasmid and nah operon is the problem).
It also looks like nah operon mutagenesis hasn't worked. We'll put this on hold, and continue with ligation of p29nah into oriT_p17c once desalting paper arrives.
Also, we'll have to redo things to confirm p39-41, SAL2 in JG700. What was done over the weekend looks weird.
August 13th, Monday
Dylan ran four simultaneous Phusion PCRs with an annealing temperature of 67degC and an extension time of 45sec to amplify mtrE. The gel looked good, with no mispriming and one band ~2.2kb. Claire did PCR clean up of the product, which will eventually be cut and ligated into p31c (see: strain list).
Because the results from the weekend's screenings were confusing, we also ran PCRs of p39-41k (miniprepped from JG700 strains) to confirm whether conjugation into Shewanella was successful. These plasmids contain mRFP under the control of constitutive promoters of varying strengths. After confirmation, we will use these parts to characterize our inducible promoters in Shewanella.
Dylan also started a liquid culture of JG700 + SAL to be inoculated into a continuous flow reactor with M4 media. With this setup, we will begin characterizing our reporter strains, initially in response to salicylate.
Dylan performed a Phusion PCR to amplify mtrE, and ran a gel to check for product. There was a single band so no mispriming was occurring.
Claire performed a double digest of the p14k and p16k with XbaI and PstI HF. This is for the construction of our final biobricks to be submitted to the registry.
August 14th, Tuesday
Steven performed a double digest of mtrE (EcorI & SpeI), Sal (SpeI & PstI), Sal2 (SpeI & PstI), and p26a (SpeI & PstI).
Steven performed a ligation of p29nah + oriTp17c, and also a ligation of p27 digest + Sal. Dylan transformed colonies with the ligation.
August 15th, Wednesday
Dylan ran a gel and Claire performed gel extraction of mRFP to put downstream of Sal reporters. They also checked to see if site-directed mutagenesis was successful for the nah-p31c. Found the digest of mRFP and p26 looked good, but the mutagenesis looked strange.
Dylan performed a taq PCR to confirm the presence of Sal2 in Shewanella. However no band was present in the gel ran afterwards, so perhaps colony PCR is the next best step.
Dylan also ran a gel of the p14k, p16k p31 digests (all cut with XbaI and PstI) for the set-up for forming the biobricks.
Steven performed a colony Phusion PCR of four colonies containing the p4k plasmid to again test for the presence of mtrE. Spencer then did PCR clean up
Spencer performed a double digest of p4kdlg and p27a cutting with EcoRI & SpeI followed by running a gel and extracting the digests.
Spencer picked colonies from the previous ligations of the Sal, Sal2, p31c biobrick plasmids for miniprepping on Thursday.
This morning Dylan noted that we may be getting a detectable basal level of current - around 6mA - in a continuous flow setup. This would make it possible to bypass the addition of mtrE to the salicylate sensing part, and still be able to distinguish Shewanella not detecting salicylate from dead Shewanella. He then added salicylate to the reactor innoculated with our salicylate reporting strain, bringing the concentration to 10uM. Later in the day, he noted that the current had risen to around 9mA, which is promising: our strain may be working! Claire set up four hour digestions with XbaI and PstI to put our arsenic parts, p14 and p16, into the iGEM backbone for submission.
Dylan also transformed a salicylate reporter with mRFP downstream into DH5alpha and WM3064, as well as the nah operon with the p29 promoter in a p17c backbone with an oriT. The second of these could be conjugated into Shewanella if transformed successfully. The first, the salicylate reporter with mRFP downstream, is a fluorescent version of the salicylate reporter. The fluorescent versions of our reporters is an alternative to qPCR to measure the relative expression level of mtrB: if we know what mRFP under the control of a constitutive promotor in Shewanella looks like, we can add arsenic or naphthalene/salicylate to our fluorescent reporter parts until reaching the same level of expression. Then, we can correlate this concentration of arsenic or salicylate to a certain strength of induced expression.
In case the fluorescent SAL reporter was not successfully ligated, we are preparing more mRFP, SAL, and SAL2: - In the morning Claire also cleaned up the digestions of SAL, SAL2 and mtrE PCR. However, due to a silly mistake on her part involving wash buffer and absolute ethanol (absolutely missing, to be precise) we will redo the digestions in order to get more DNA for putting these reporters into the iGEM backbone, and to put mRFP downstream. She then set up four hour digestions with XbaI and PstI to put our arsenic parts, p14 and p16, into the iGEM backbone for submission. - We digested mRFP with SpeI and PstI to make the part with mRFP downstream of mtrB in the salicylate reporters. We have already successfully put mRFP downstream of mtrB in our arsenic reporters, but it did not seem to work in the arsenic reporter. The gel for mRFP looked as expected, with the band for the insert slightly shorter than 900bp. However, on the same gel the the mutagenesis trial of the nah operon, digested with PstI and NotI, showed only one band. If mutagenesis had not worked, we would expect more than two bands, and if it had worked we would still expect two bands. Therefore we are unsure how to interpret this result, which we have seen twice now, but are going to start over with site-directed mutagenesis of the nah operon. We will also visualize the unmutated nah operon, digested with PstI and NotI, on a gel to see if that looks like we expect it to.
Finally, it should be noted with jubilance that Claire was reunited with her umbrella today! The joy in the lab at this event was palpable and will be remembered for years to come.
August 16th, Thursday
Dylan performed a miniprep of the Sal, Sal2, and p31c reporters for us to do sequencing for confirmation of successful ligations. These will hopefully be the biobricks we send in.
In the afternoon Dylan performed a dephosphorylation of the p31c (cut with XbaI, PstI) followed by a gel of the mtrE cut with EcoRI & SpeI as well as mRFP cut with SpeI and PstI.
A 4 hour digestion was also performed by Claire to get Sal parts into pSB1C3 for the SAL, SAL2, and p31 parts. Steven then ran a gel of the digest products.
Steven also performed a colony Taq PCR of the Sal2 reporter to confirm its presence in Shewanella.
Today, we continued work to get our engineered reporters into pSB1C3 for submission to the parts registry. In the morning, Dylan miniprepped both versions of our salicylate reporters, along with more pSB1C3 from overnight cultures of the corresponding DH5a strains. Following quantification, he set up digestions of each miniprep with XbaI and PstI. (We decided to cut with XbaI because we discovered an extra base pair between the NotI and XbaI cutsites in the normal BioBrick prefix, an artifact of a previous team's work). Caleb also dephosphorylated previously isolated pSB1C3 backbone to prevent self-ligation, while Steven gel extracted the salicylate reporter inserts and more pSB1C3 backbone (to be quantified Friday morning).
August 17th, Friday
Claire performed a ligation for the construction of the Sal_RFP, and Sal2_RFP plasmids. Transformation was then performed immediately after.
August 18th, Saturday
August 19th-25th
Click to view the details.
August 19th, Sunday
In the morning, Dylan came to lab to look at the transformant plates from Saturday. There were colonies on all but one of the plates: It looks like either the ligation to put mtrE into p31c or the subsequent electroporation failed. It is of note that the competent DH5α cells that Dylan used for the mtrE electroporation were from an older stock – one which we'd previously determined to confer lower transformation efficiency. Because we used up all of the newer stock – with markedly better efficiency – we will make more electrocompetent DH5α in the coming week.
Because all other transformations seemed to work, we grew up overnight cultures from single colonies on each plate. We will miniprep from these cultures and screen the minipreps for the correct insert. If confirmed, we will have successfully have put both versions of our arsenic reporters, as well as both versions of our salicylate reporters inside p31c (i.e., pSB1C3), as is required for registry submission. We will also have successfully put mRFP downstream of mtrB on SAL2, which will be used for characterization of the salicylate sensitive promoter in S. oneidensis.
August 20th, Monday
August 21st, Tuesday
August 22nd, Wednesday
Conjugating nah into Shewy: Caleb, Tina, and a guest Louis miniprepped 5 plasmids containing nah operons with constituitive promoters from a WM3064 plate and miniprepped one plasmid that we previously confirmed as having the nah operon (without a promoter) to use as a positive control in the future.
Claire performed miniprep of grown cultures of the Sal_RFP and Sal2_RFP to submit for sequencing.
August 23rd, Thursday
August 24th, Friday
Jim performed the digestion of the nah operon+ p29a and the p31c backbone for ultimate ligation in the creation of the last biobrick. Ligation had to be redone, as sequencing results of last week's ligations looked great except for the nah operon biobrick.
Conjugating nah into Shewy:
We wanted to accomplish two things:
- Amplify the nah operon with a promoter using primers with appended cut sites.
- Optimize colony PCR for future Cornell iGEMers (past attempts have failed.)
To accomplish both of these objectives, we did Phusion PCRs using plasmids containing nah operons with constituitive promoters as a template and a Phusion colony PCR using the same colonies we miniprepped the plasmid from. Because we expect the miniprep PCRs to work, doing the colony PCRs in parallel will allow us to determine whether our colony PCR technique is working or not. For our colony PCR purposes, we want to be able to screen for colonies while being certain we can go back and miniprep the same PCRed colony. Our colony PCR method is as follows:
NOTE: We later determined there was a cataclysmic flaw in the following method, can you figure it out? (wink wink nudge nudge...)
- Label colonies of interest and correspondingly label microfuge tubes
- Add 50 uL ddH2O to each microfuge tube
- Using pipette tip, dip into colonies of interest then dip into labelled microfuge tubes and swirl around to release cells
- Use the the ddH2O+cell mixture as the DNA template for a PCR - instead of adding plain ddH2O to the PCR to bring it up to volume, add the ddH2O+cell mixture
- Confirm your PCR had desired results with a DNA gel, then use the ddH2O+cell mixture from the corresponding microfuge tube the PCR was done on to inoculate a culture or plate.
August 25th, Saturday
Jim performed an overnight ligation of the nah operon pSB1C3 for transformation on Sunday.
Conjugating nah into Shewy:
Caleb, Tina, and a guest Louis ran a gel of the miniprep and colony parallel Phusion PCRs. All of the miniprep PCRs worked, but only a couple colony PCRs worked. We believe this is due to not having added enough cells to the colony PCR tube because some of the colonies were very tiny.
August 26th-31st
Conjugating nah into Shewy: The nah operon was ligated to a backbone containing an origin of transfer in a ratio of 3:1 and 1:1. The ligation was electroporated into WM3064. Unfortunately, cell growth was extremely slow, and after colony PCR, we discovered that the PCR failed due to the lack of the positive control on the gel...but why? Caleb had a brilliant thought, leading to a modification of the colony PCR protocol - instead of using the cells and ddH20 as template, which may cause the cell to lyse due to hypotonic environments, we now will dip the pipette tip into the colony, THEN dip and swirl it in the PCR reaction tube, THEN swirl the same pipette tip into a microfuge tube with LB! Colony PCR and overnight cultures in one go! Genius Caleb! Unfortunately, after performing colony PCR with the newly modified protocol, the positive control appeared - but the test colonies lacked bands in the proper places. Time to re-do ligations.
Site-directed mutagenesis: This week, Swati re-initiated efforts to mutate the three internal cutsites of the nah operon, which consist of a mutagenic PCR step, a digestion with DpnI to chew up excess, non-mutated template. Most of this week consisted of troubleshooting.
August 26th, Sunday
Conjugating nah into Shewy:
Caleb miniprepped two plasmids - one contained the nah operon preceded with a constitutive promoter and the other is to become a backbone containing the origin of transfer required for conjugation and a gene conveying chloramphenicol resistance. The purified plasmids were digested to extract the nah operon and to cut open the backbone - these digests were then run on a gel. Tina gel extracted the backbone then accidentally threw away the rest of the gel that contained the nah operon fragments.
Site-directed mutagenesis: Swati ran a mutagenic PCR of nah_p31c, then digested with DpnI to remove template DNA and column purified the resultant DNA.
August 27th, Monday
Conjugating nah into Shewy:
Caleb gel extracted the nah operon containing fragments from yesterday - despite having been in the garbage overnight, the gel extraction worked! Caleb and Tina began a 24 hour 16 degrees Celsius ligation using molar rations of nah to backbone of 3:1 and 1:1.
Fluorescence tests: Claire started a JG700 + SAL2 culture and tried to do a PCR of SAL2_mRFP with sequencing primers to see if the band produced would correspond to the length of SAL2 plus the length of mRFP. Unfortunately, due to a mishap involving p10s vs. p2s and not checking what volume her pipette was set to, she ended up with a 70uL total volume and PCR failed.
Site-directed mutagenesis: Swati transformed the (hopefully) mutated DNA into DH5a.
August 28th, Tuesday
Conjugating nah into Shewy:
Caleb and Tina de-salted the 3:1 and 1:1 ligations, then transformed them into a DAP-requiring conjugation strain WM3064 via electroporation. The electroporated cells were plated on DAP + Cm plates.
Fluorescence tests: JG700 once again takes a long time to grow, so it wasn’t until the evening that Claire miniprepped SAL2 from JG700. Tomorrow we will run another PCR and see if we have SAL2_mRFP, and SAL2 in JG700.
Site-directed mutagenesis: Swati's transformation did not work. After checking the PCR product and a PstI digest thereof on a gel, she discovered that the PCR was not working with high enough efficiency to produce a band on a gel. Upon consulting with Didi, our trusty post-doctoral adviser, she then restarted the mutagenic process with a PCR; this time, with a lower primer concentration and lower denaturation and annealing times.
August 29th, Wednesday
Conjugating nah into Shewy:
Caleb checked the transformation plates after ~14 hours in a 37 degrees Celsius incubator and noticed colonies, but the colonies were barely visible indicating very slow growth of the cells.
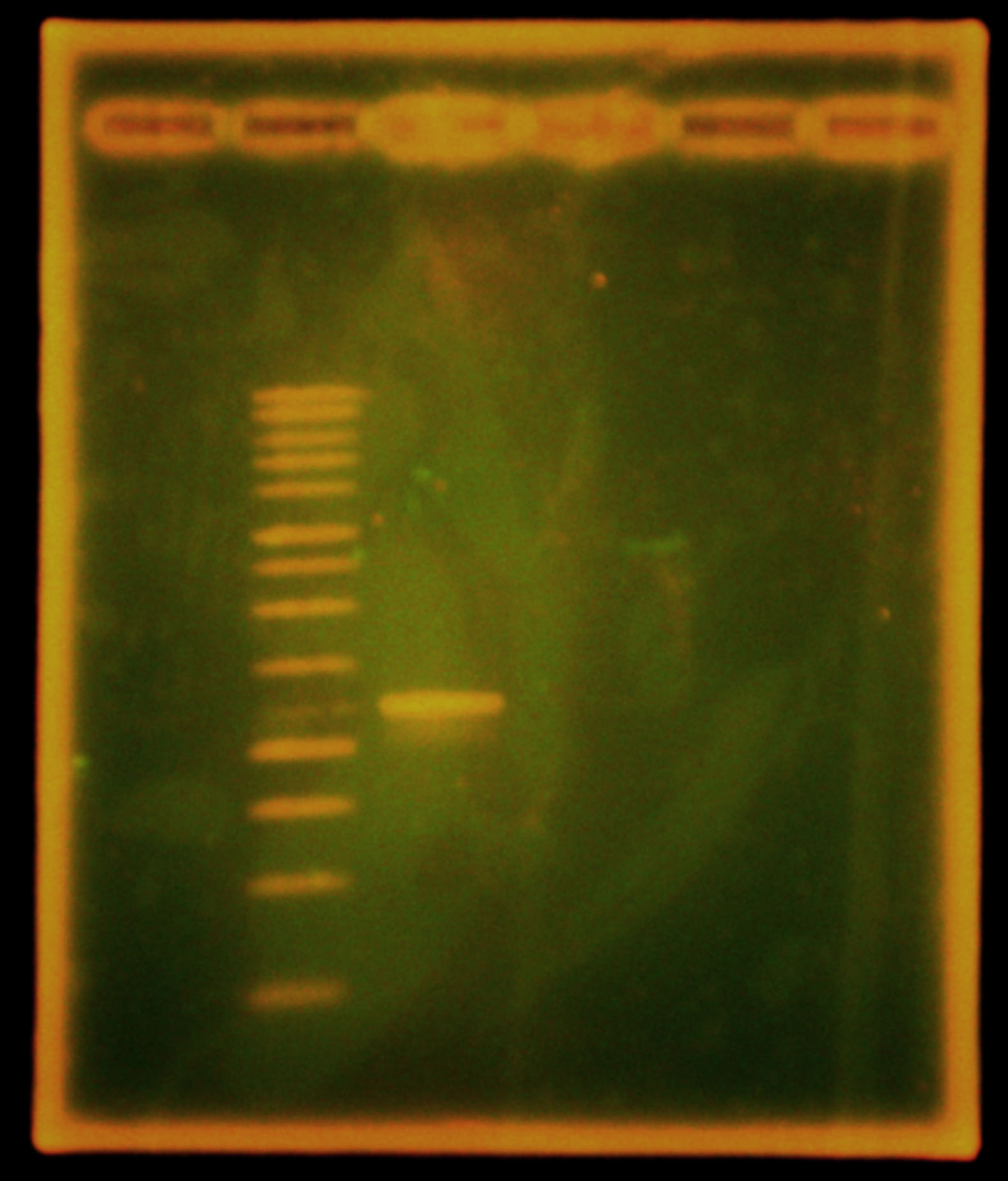
Fluorescence tests: Set up a Taq PCR of SAL_mRFP, SAL2_mRFP and SAL2, with an extension time of 4.5 minutes and annealing temperature of 68degC. SAL2_mRFP has a band corresponding only to the length of SAL2, without mRFP, so we need to redo this ligation. However, the band from SAL_mRFP was the correct length (a little more than 4kb), so this ligation was successful and we can conjugate into Shewenella. Finally, the JG700 miniprep is the correct length for SAL2 (~3.8kb), so SAL2 has been successfully conjugated into Shewenella!
Site-directed mutagenesis: Swati digested her second mutagenic PCR with DpnI, column purified, and transformed into DH5a.
August 30th, Thursday
Conjugating nah into Shewy:
Tina checked the growth of yesterday's colonies and tried doing a colony PCR of each colony and a PCR of a positive control (same product, same primes, but in a different purified plasmid). The primers were designed to sit on the backbone and face into the oriT region and the site where we wanted to insert the nah operon. A PCR amplicon indicating successful ligation of the nah operon into the backbone would be approximately 10-11kb, while a failed ligation would yield a ~700 bp amplicon. Caleb later ran a DNA gel of the colony PCRs and nothing but smears showed up in every lane of the gel except the positive control which didn't even have a smear (Caleb was so disappointed that he didn't post the picture). Since the positive control didn't show up, we concluded the PCR failed. Caleb started overnight cultures of the remaining cells+ddH2O used for the colony PCR.
Site-directed mutagenesis: Alas, Swati's second attempt at mutagenesis was yet again fruitless. Concerned that the template may have been causing the problem, she performed a NotI digest and ran it on a gel alongside the latest mutagenic PCR. The template was fine; the PCR was, again, invisible. Swati grew extremely puzzled as to how her purified PCR product could have significant concentration upon quantification but yield no visible bands on a gel. She decided to try a third time, this time with longer denaturation and annealing times, but still the same concentration of primers.
August 31st, Friday
Conjugating nah into Shewy:
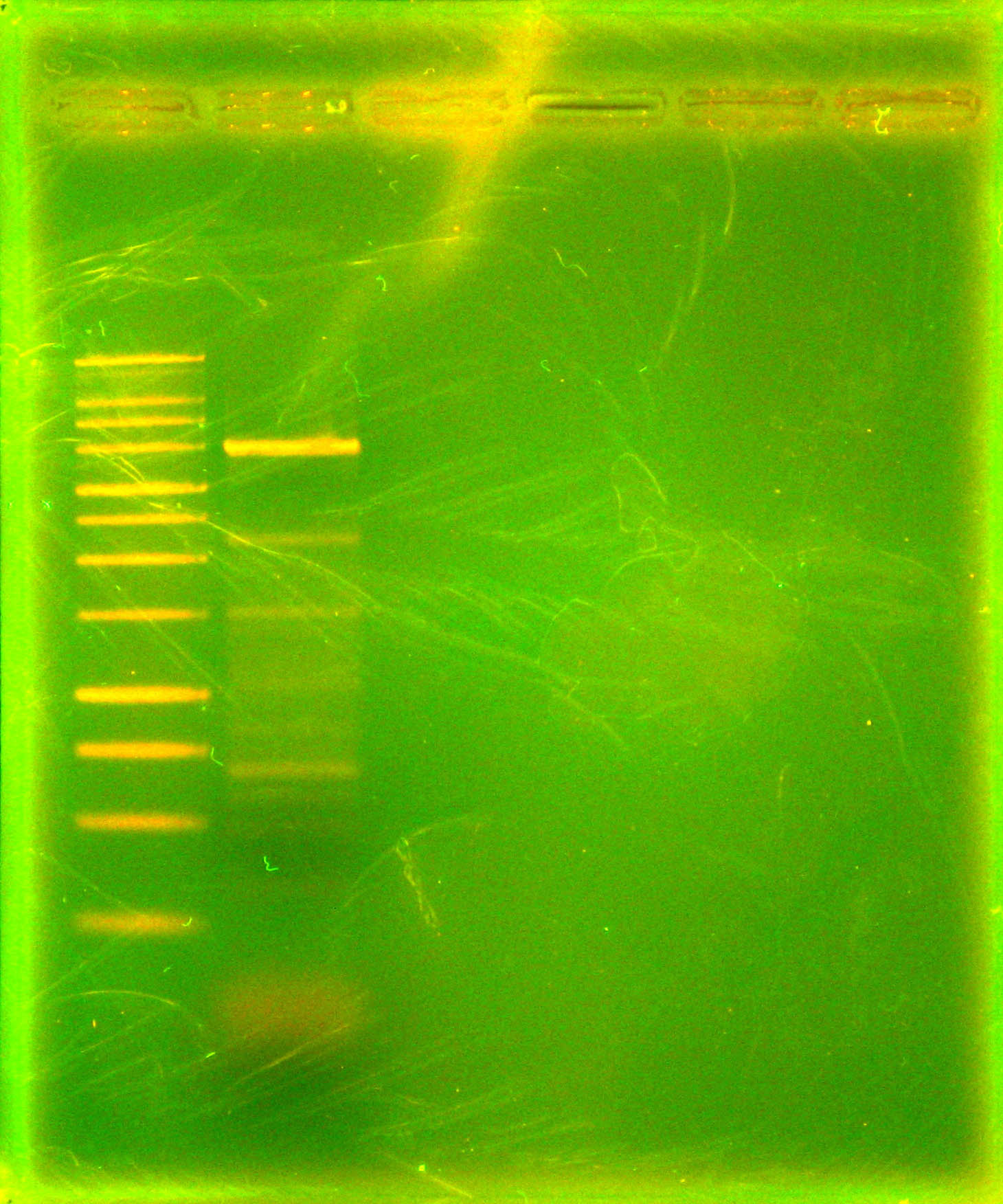
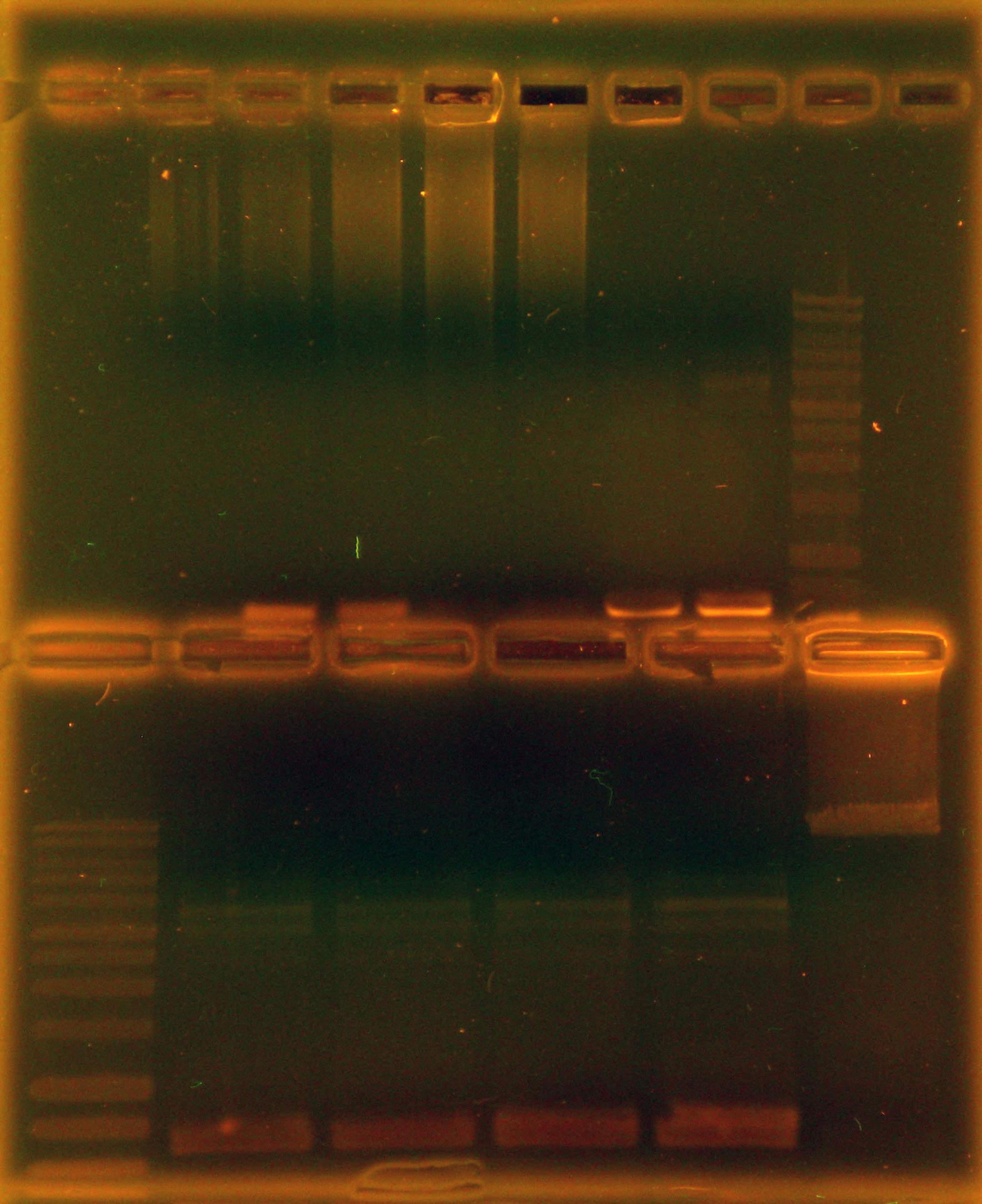
Caleb noticed the overnight cultures didn't grow and concluded the cells must have died due to the cells being stored in an extremely hypotonic solution (ddH2O). He re-did colony PCRs of the colonies, a colony with a positive control, and a positive control of purified plasmid, but modified the colony PCR protocol. Instead of using the cells + ddH2O as template DNA, Caleb dipped the pipette tip into the colony, then dip and swirled it in the PCR rxn tube before dip and swirling into labeled microfuge tubes with 50 uL LB media. The microfuge tubes were stored in a 4 degrees Celsius fridge. A DNA gel of the colony PCRs showed that the ligation of nah into the backbone failed - as seen in the accompanying gel picture, our positive control worked (indicating the PCR conditions were fine), but the test colonies all only had 700 bp amplicons.
Fluorescence tests:
Claire found some old SAL2 and mRFP digested with PstI and SpeI already, so she decided to retry the ligation. She did a 40min ligation at room temp, and continued it overnight in the 4degC fridge, which the NEB rep said could increase efficience. However, since she forgot to dephosphorylate SAL2, we will never know if this room temp and overnight in the fridge combo would have been effective because it self ligated.
Site-directed mutagenesis: Swat ran a DpnI digestion of half of the previous day's PCR product, in an attempt to rule out the possibility that DpnI was functioning incorrectly.
September
September 1st-8th
Conjugating nah into Shewy: The mysterious failure of past week's ligation lead to a redigestion (in case we run out of DNA) and religation of last week's digestions. Nah to oriT was ligated in ratios of 3:1 and 6:1 - when run on a gel, bands once more failed to appear... But wait! Epiphany! The team realized that we forgot to dephosphorylate the oriT backbone! After that silly mistake, 1:1, 3:1, and 6:1 ratios of nah:oriT were set up in a ligation mixture and electroporated into WM3064. Once again, slow growth and tiny colonies were observed - perhaps due to the strain of putting such a massive operon in a cell. 13 colonies were deemed large enough to try colony PCR, and 3 of them definitely showed bands that corresponded to the length of our construct. We set up liquid cultures for miniprepping and once again observed slow growth. We tried subculturing - but still, general opaqueness. Miniprepping proceeded anyway, as we are a fairly optimistic bunch (and the cultures were by then over 24 hours old).
Site-directed mutagenesis: After several more unsuccessful attempts, lengthy discussion among teammates, and consultation with several graduate advisors, the team decided to put this subproject on hold for the time being. Swati finished up with one last attempt, then joined Claire for fluorescence testing. That's all, folks!
September 1st
Conjugating nah into Shewy:
Due to the mysterious failings of our attempts to ligate the nah operon, Tina tried to start fresh by re-digesting the nah operon-containing plasmid and the oriT-containing backbone.
Synthetic River Media:
Mark and Chie created synthetic river media based on information on the mineral content of the Athabasca River, and started overnight cultures of numerous strains to test how sodium lactate supplemented river water would grow the cells.
Site-directed mutagenesis: Swati transformed both her mutagenic PCR and her DpnI digest into DH5a after desalting on a membrane. She also ran both of the aforementioned samples on a gel without purifying, in order to check if either the digestion or purification steps were problematic. The gel was yet again blank, suggesting that the PCR itself was the problem.
September 2nd
Conjugating nah into Shewy:
Tina tried ligating an older digestion of the nah operon into the oriT backbone using a molar ratio or 3:1 and 6:1 for nah:backbone. She then de-salted and transformed the ligations into conjugation strain WM3064. Tina then ran a gel of the digested nah operon-containing plasmid and the oriT-containing backbone and extracted the nah operon and oriT backbone bands.
Synthetic River Media:
Mark set up a 96-well plate to test growth of the engineered strains in sodium lactate at varying concentrations, in synthetic river media.
Site-directed mutagenesis: The third transformation attempt yielded no colonies on the plate of DpnI-digested DNA, but three colonies on the PCR only plate, suggesting that these were just excess template. Nonetheless, Swati set up cultures to miniprep the following day. She also realized that she had been using a template that was 10x more concentrated than it was supposed to be. She proceeded to set up a fourth attempt at a mutagenic PCR, this time with the correct template concentration, primers for mutating a different cutsite than she had previously been attempting, and a higher annealing temperature.
September 3rd
Conjugating nah into Shewy:
Tina extracted the nah operon digest and the oriT backbone from yesterday's gel. Tina and Dylan had an epiphany - we forgot to dephosphorylate the backbone! No wonder we kept seeing self-ligations! With a renewed spirit, Tina dephosphorylated the digested oriT backbone then started a sixteen hour 16 degrees Celsius ligation of the extracted digests. Tina tried nah to backbone ratios of 1:1, 3:1, and 6:1.
Synthetic River Media:
Mark returned to the lab to discover that the growth assay went wrong. Numerous issues were identified with the original protocol to explain the failed result, and the protocol was changed.
Site-directed mutagenesis: Swati miniprepped the three suspicious PCR only colonies from the previous day, and digested with PstI to check for successful mutagenesis. She also did a DpnI digest and transformation of the PCR from the previous day. She ran the new PCR & DpnI digest, along with the PstI-digested minipreps of the three earlier colonies, on a gel, but to no avail. The new things showed nothing (again), and the digested minipreps produced completely incorrect bands.
September 4th
Conjugating nah into Shewy:
Tina desalted yesterday's ligations then transformed them into our conjugation strain WM3064. WM3064 is E. coli that is auxotrophic for DAP (diaminopimelic acid epimerase).
Site-directed mutagenesis: The latest transformation did not work. Thus concludes this subproject!
September 5th
Conjugating nah into Shewy:
Caleb and Tina checked the transformation plates - some colonies were visible on all three ligation ratio pltaes, but the colonies were so tiny we decided to wait another day before trying colony PCRs or starting liquid cultures.
September 6th
Conjugating nah into Shewy:
Caleb decided the transformation colonies were large enough to colony PCR and start liquid cultures from. 13 total colony PCRs were performed on colonies from all three ligation mixtures and one positive control was run in parallel to be sure the PCR conditions were correct. Tina ran a gel of the the colony PCRs and the poitive control.
As can be seen in the gel pictures, colonies 3, 4, and 8 definitely had the nah operon and many other colonies had matching middle bands. These matching middle bands likely indicate some sort of mispriming. Colony 5 was clearly the product of an oriT backbone self-ligation. Caleb and Tina started 15 mL liquid cultures of apparently nah containing colonies 3, 4, and 8 and non-nah-containing colony 5 to miniprep from tomorrow.
Fluorescence tests: This week started late as Claire was getting over a massive cold. However, with new vim and vigor and her trusty labmate Swati, they set forth to decide the details of how they will run fluorescence tests. To get ready for this, they set up cultures of JG700 and E. coli constitutively producing mRFP under the control of Anderson series promoters.
September 7th
Conjugating nah into Shewy:
Overnight cultures from yesterday looked slightly opaque, but not opaque enough to miniprep from, so Caleb subcultured the cultures hoping the cells would divide fast enough to be ready for miniprepping tomorrow.
Synthetic River Media:
Mark started overnight cultures of all engineered strains to test, again, how growth in synthetic Athabasca river water supplemented with sodium lactate.
Fluorescence tests: JG700 and p41k didn’t grow, so we decided to just use E. coli do decide what experimental conditions would be best. We tried both seeding the plate with 50uL of different OD’s of constitutively produced mRFP too see what the optimal OD to start at is. Our results were strange, though – we decided that 50uL is not a large enough volume for getting accurate OD readings, but that using a larger volume (100uL) of sample at an OD around mid-log phase (~0.5 or 0.6) would make the most sense.
September 8th
Conjugating nah into Shewy:
The subcultures of colonies 3, 4, and 8 were not as opaque as Caleb was accustomed to miniprepping from (colony 5 was very opaque, though), but he went ahead and miniprepped anyway because the cultures were already more than 24 hours old. General convention is to miniprep E. coli cultures after ~16 hours.
Synthetic River Media:
Mark and Chie prepared a new growth assay of all strains, and allowed a full day time course of growth to be taken.
September 9th-15th
Conjugating nah into Shewy: Unfortunately, the miniprep yields from last week were all low except one shining colony - which didn't have the nah operon in it. We ran it to test our hypothesis that slow growth of E Coli was due to the added stress on the cell of having such a large operon in it - our data so far matches our hypothesis, as that colony grew faster in culture and the plate and also yielded substantially more DNA after miniprepping.
Though we were unable to confirm the presence of the nah operon with sequencing (as the miniprep failed), we decided to proceed with conjugation into Shewanella just in case the transformation succeeded. We tried to grow one of the colonies with a very strong band from last week's gel in a liquid culture, and once again, observed slow growth. We conjugated into JG700 and JG700 with SAL, a plasmid with our salicylate reporter system. Liquid cultures were made from the conjugation - unfortunately, there was no growth. We streaked new plates with the old transformation in the hopes that something would finally grow!
September 9th
Conjugating nah into Shewy:
Caleb checked the miniprep yields today - all but colony 5 had yields below 90 ug/uL. Colony 5 had a yield of over 500 ug/uL. Our hypothesis as to why the E. coli was growing so slowly was that the E. coli was stressed from having to express the many nah operon proteins. Evidence suuporting our hypothesis includes - colony 5 had a much larger colony, grew in liquid much faster, came out as lacking the nah operon, and yielded a lot more DNA from miniprepping than the colonies that supposedly had the nah operon.
Synthetic River Media:
Again, the result of the growth assay was failure. This time, it appeared that the only issue was blanking, as the negative controls were reading higher than the inoculated samples. After some discussion with Dr. Archer, a better blanking method was determined, as well as the addition of longer mixing to the protocol.
September 10th
Fluorescence tests: We dephosphorylated SAL2 digested with SpeI and PstI for one hour, then heat killed at 65degC for 10min. We then ligated overnight with mRFP (also digested with SpeI and PstI) at 16degC in the thermocycler.
However, JG700 and p41k didn’t grow, so we moved the plates to Riley Robb in the hopes that a more controlled incubator meant for Shewenella will help them thrive.
September 11th
Fluorescence tests: Swati transformed 2uL of SAL2_mRFP ligation mixture into WM3064. We also made cultures of JG700, WM3064, and p39k, p40k, and p41k in both JG700 and WM3064. We are hoping to run a plate with both strains on it to get an idea of whether we can see mRFP constitutively produced in JG700 (which is naturally red and may have some background signal), and compare that fluorescence to constitutively produced mRFP in WM3064. Once we have this control data we hope to be able to correlate fluorescence in JG700 with promoter strength.
September 12th
Conjugating nah into Shewy:
Caleb and Tina started cultures of colony #3 from original transformation plate and cultures of JG700 + SAL and JG700. JG700 is Shewanella oneidensis with a ΔmtrB genotype. SAL refers to a plasmid with our reporter system that responds to the presence of salicylate by upregulating expression of mtrB.
Fluorescence tests: Cultures of JG700 p39k and p41k didn’t grow. We made new cultures with giant swabs of cells from the plates to see if the plates are dead, and subcultured from all the other cultures started yesterday.
There are also two colonies on our SAL2_mRFP transformation plate, so we made 25mL cultures from these to see if we ligated successfully.
September 13th
Conjugating nah into Shewy: Caleb started liquid cultures of colonies #3 and #4 from the original transformation plate. Caleb also started a conjugation of JG700 + SAL with colony #3 and JG700 + colony #3. The conjugation was at room temperature on a DAP plate for 16.5 hours.
Fluorescence: Swati miniprepped the SAL2_mRFP cultures, and Claire set up a PCR to confirm whether SAL2_RFP was ligated successfully. Unfortunately it doesn’t look like the ligations worked, as neither band is ~4.5kb, which should be the length for SAL2 with mRFP. We think that the old digestions we are using may not be good, so we will start from scratch next time.
September 14th
Conjugating nah into Shewy: Caleb checked the liquid cultures from yesterday and noted there was no growth, as expected, due to the nah operon stressing the cells. Caleb streaked new plates from the conjugation - the LB agar plates had kanamycin and chloramphenicol for the JG700 + SAL and colony #3 conjugation and just chloramphenicol for the JG700 and colony #3 conjugation. The plasmid in the SAL strain confers kanamycin resistance and the plasmid in colony #3 with the nah operon confers chloramphenicol resistance. The strain of E. coli in colony #3 was auxotrophic for DAP, so the E. coli couldn't grow on the newly streaked plates.
Synthetic River Media: Cultures of only JG700 and Sal1 containing cells were started today, for another attempt at the growth assay.
September 15th
Running Reactors: Because maximal current production did not increase, Dylan took down reactors with working electrodes poised at 0.35V with respect the the Ag/AgCl reference electrode in order to free up potentiostat channels so that we can begin characterizing our arsenic reporter strains.
September 16th-22nd
Conjugating nah into Shewy: Some colonies were observed from last week's restreaking! A massive 15 colony liquid culture was set up, but to no avail. After five long days, we were forced to conclude that conjugation failed. Sadface. Good thing we had extra 3:1 nah to oriT ligation sitting around, which we transformed into both WM3064 and DH5a, the latter of which we hoped would grow faster. 16 colonies from the Sep 4th ligation were also restreaked. And lo! Every single restreak had colonies the next day, which warranted a massive 16 colony colony PCR (try saying that five times fast). Interestingly, the WM3064 plates grew, but the DH5a transformation failed, which we concluded was due to a bad electrocompetent cell stock (we noticed weird white precipitate in the cell mixture before).
September 16th
Conjugating nah into Shewy:
Caleb checked the streaked conjugation plates - there were some colonies, but they were all on top of the initial streak. He and Tina started liquid cultures of all 15 colonies.
Synthetic River Media:
After finalizing the growth assay protocol, another experiment was set up, this time with only two strains (JG700 and Sal1) in triplicate with sodium lactate concentrations (varying from none to full M4 media concentrations) in synthetic Athabasca river water, plus positive LB controls and blanks. The assay was set to run at room temperature for 20 hours, taking data every 5 minutes with 30 seconds of mixing before each read. Blanking was done by group, for each media mixture.
Fluorescence tests: Started liquid cultures of JG700, p25a, and p29a in order to re-conjugate these constitutively produced mRFP plasmids into Shewanella. p25a on a pBBRBB backbone in WM3064 corresponds to p39k in JG700, and p29a corresponds to p41k.
September 17th
Running Reactors: After autoclaving a reactor setup and getting peristaltic pumps ready for a new continuous flow experiment, Dylan started a liquid culture of an arsenic reporter strain (JG700 + p14k) to be inoculated into reactors.
Conjugating nah into Shewy:
Caleb checked the liquid cultures started yesterday - no growth.
Synthetic River Media:
The growth assay was successful, with constant and low OD600 in the blank wells, and a standard growth curve in the LB wells. The data suggests that lowering the sodium lactate from M4 concentrations is impossible. Therefore, a solution containing 5% volume/volume of 40% sodium lactate weight/volume is what is necessary to maintain an OD600 of approximately 0.1 for an extended period of time. However, the supported OD600 may be higher in the final device at this concentration, due to the constant flow of new sodium lactate.
Fluorescence tests: We redigested SAL2 and mRFP with SpeI and PstI-HF to try to clone mRFP into our salicylate reporter, so that we can do fluorescence experiments with the two salicylate parts. Swati also plated WM3064 with JG700 for conjugation of the constitutively produced mRFP.
September 18th
Conjugating nah into Shewy:
Caleb checked the liquid cultures started on the 16th - no growth.
Fluorescence tests: We ran mRFP on a gel and extracted a 800bp band which was the appropriate link for mRFP. After enzyme purifying the SAL2 digestion and dephosphorylating, Claire set up an overnight ligation of SAL2 and mRFP.
September 19th
Conjugating nah into Shewy:
Caleb checked the liquid cultures started on the 16th - no growth.
Fluorescence tests: Claire transformed the SAL2_mRFP ligation into WM3064, and set up cultures of p39k and p41k from the conjugation plate.
September 20th
Conjugating nah into Shewy:
Caleb checked the liquid cultures started on the 16th - no growth.
Fluorescence test: Swati miniprepped cultures of p39k and p41k from the conjugation plates so that we can PCR to confirm if we have successfully conjugated constitutive mRFP into Shewanella.
September 21st
Conjugating nah into Shewy:
Caleb checked the liquid cultures started on the 16th - no growth. He concluded that the conjugation failed. Caleb and Tina electroporated the 3:1 (nah:oriT backbone) ligation of dephosphorylated and digested nah with the oriT backbone from September 3rd into both WM3064 and a DH5α strain. They then streaked 16 colonies from the September 4th transformation onto fresh plates.
September 22nd
Conjugating nah into Shewy:
Caleb and Tina noted the appearance of the re-streaked plates - every single streak had numerous colonies. They started liquid cultures and started an overnight colony PCR of all 16 of them. Included with the colony PCRs was a positive control that we knew would have the same amplicon from the nah operon if the colonies were the result of a successful ligation. The electroporated WM3064 plate grew with numerous isolated colonies; the DH5α transformation failed. We hypothesized the DH5α cells were at fault due to their appearance before electroporation - white precipitate was floating in the cell mixture.
Fluorescence tests: Using samples from the 20th, Swati did a Phusion PCR to see if we successfully conjugated p39k and p41k into Shewanella. She also miniprepped WM3064 SAL2RFP and did PCR to see if that ligation was successful.
September 23rd-30th
Conjugating nah into Shewy: A gel of last week's colony PCRs hinted that one of the colonies had the nah operon in it! Excitement! Liquid cultures were made, and there was just enough DNA after miniprepping to submit for sequencing. We will collectively hold our breaths and wait for positive results.
This week, we were able to begin characterizing the current response to arsenic of S20 (JG700 + p14k; see strain list). Additionally, we submitted physical DNA for six BioBrick parts to the parts registry.
September 23rd
Conjugating nah into Shewy:
Caleb and Tina ran a gel of the colony PCRs and positive control PCR.Fluorescence tests: All of our conjugations and ligations look good! We can now run our control plate, and will sequence SAL2_RFP to see if we will be able to do salicylate reporter testing as well. Swati also started cultures of our arsenic-sensitive strain, as well as control strains, for a plate testing our arsenic reporters.
September 24th
Conjugating nah into Shewy:
Caleb inoculated 30 mL LB+DAP+Cm with the 24 hour liquid culture of of colony N.
September 25th
Fluorescence tests: Swati set up a conjugation of SAL2_mRFP into JG700. Also, sequencing came back good so once we have this part in Shewanella we can start testing our salicylate sensor. Jim and Swati also set up a plate to test our arsenic sensing parts at different concentrations of arsenic – they ran a plate with a blank LB column, five control columns (JG700, MR-1, p39k, p40k, and p41k), and three columns of each of our arsenic-sensitive strains. To each row they added a different concentration of arsenic, going from 0uM to 5uM arsenic. The final OD of 100uL in each well was 0.8, and the plate was left overnight in the plate reader.
Conjugating nah into Shewy:
Caleb miniprepped the 36 hour 30 mL culture of colony N. The size of the pellet after the first spin step was similar to the size of a ~5mL normal culture, consequently, the yield was only 96.7 ng/uL.
September 27th
Running Reactors: For the first time, we added arsenic to our reactors. Specifically, once Dylan saw that the two reactors inoculated with an arsenic reporter strain (JG700+p14k) had been producing steady current for several retention times, he dosed the media vessel to a final concentration of 10 μM sodium arsenite.
Conjugating nah into Shewy:
Fortunately, there was enough DNA for Caleb to submit the miniprepped DNA from colony N for Sanger sequencing today. Tina started 3 x (1mL cultures of colony N).
Fluorescence tests: Claire and Swati set up another control plate with constitutively produced mRFP in both WM3064 and JG700. Since using 100uL starting at and OD of 0.8 seemed to work well for the arsenic plate, we set up the control plate with the same parameters and left it in the plate reader overnight.
September 28th
Running Reactors: Since several retention times passed without any current response to 10 μM sodium arsenite, Dylan increased the concentration in the media vessel, hitting our reactors with 100 μM arsenite.
Fluorescence tests: We noticed that the arsenic data did not seem to have any signal above background, and realized that it may have been because the plate reader wasn’t set to read a clear-bottomed plate. We concluded that, since from the test done on the 27th we could see distinct difference between fluorescence levels of constitutively produced mRFP, our experimental procedure is good and the plate reader was just on the wrong setting. Additionally, since the bioreactors are not getting upregulation of arsenic up to 10uM, we decided next time to test higher concentrations of arsenic (0uM to 500uM)
 "
"