Contents |
The "SWITCH" Project
In a nutshell
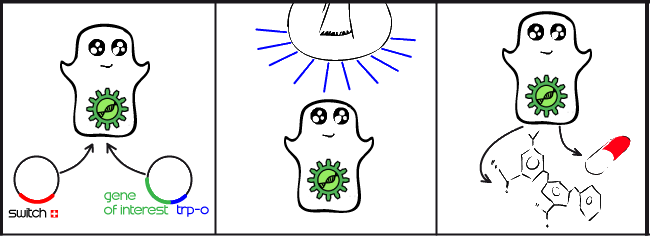
Our project is all about implementing a light activated genetic switch in mammalian cells. The production of complex proteins is becoming an increasingly commonplace task, particularly in the pharmaceutical industry. Mammalian cells are an ideal environment to make these proteins, but producing them at the right time and optimal rate requires an expression system controlled by a specific signal. Until now, these systems have mostly been limited to chemical signals which have many drawbacks. By using a light-activated expression system, we hope to enable mammalian cells to respond to a light signal, rather than a chemical signal, by initiating gene expression for a protein of interest.
A new expression system: LovTAP fusion protein
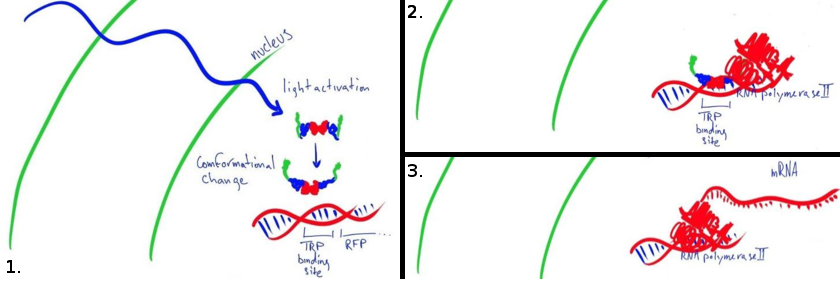
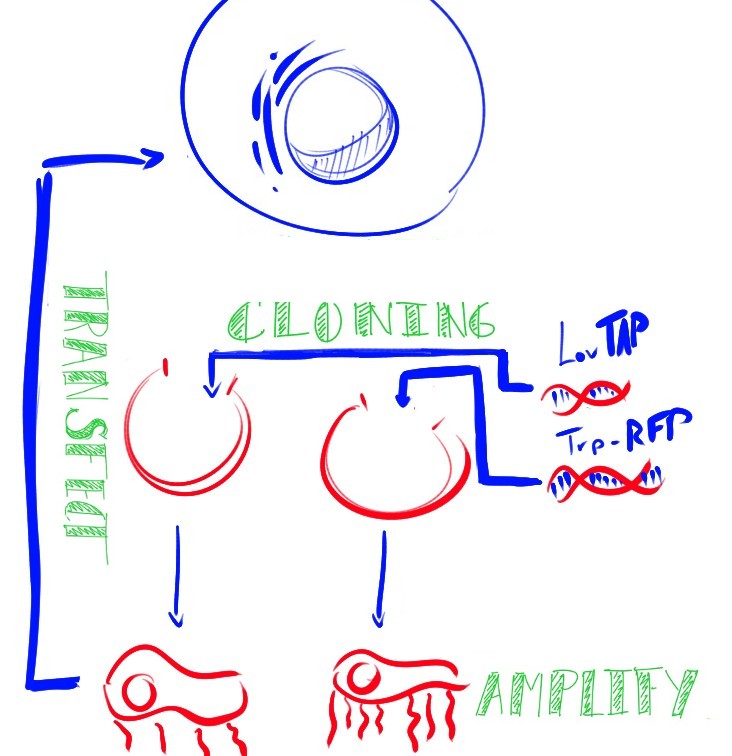
Our main goal this summer was to implement a previously untested expression system in mammalian cells. The LovTAP-VP16 fusion protein we used is based on a previously tested fusion protein, [http://partsregistry.org/wiki/index.php/Part:BBa_K191006 LovTAP] by the 2009 EPF Lausanne team, which binds to a specific sequence of DNA once it is activated with blue light. By attaching an activating domain to the protein that is known to work in mammalian cells, we hoped to enable LovTAP-VP16 to bind DNA after exposure to light and regulate expression of a gene next to its binding site in a mammalian cell line.
Another approach: Melanopsin
We also worked with another light-activated expression system that has already been proven to work. Martin Fusseneger et al. have designed a pathway that makes extensive use of gene expression pathways already present in the cell. By adding a light-activated receptor on the cell membrane, they promote the release of calcium into the cytoplasm upon exposure to light. This activates the native cellular response to an increased calcium concentration. Promoter proteins sensitive to calcium will react to this change and start expressing genes with specific promoter sites. By introducing a new gene with a calcium-sensitive promoter next to it, we can use light to induce its expression.
CHO cells & Co
CHO (Chinese Hamster Ovary) cells are the workhorses of industrial protein production. The light activated expression systems we worked on would be extremely convenient for production of proteins in an industrial context in these cells. For our project, we transfected these cells with each expression system and conducted a variety of tests. Along with the CHO cell line, we also chose to test these light activated systems using HEK (Human Embryonic Kidney) cells since the melanopsin system had already been shown to work in this cell type. The melanopsin system would provide data to compare to our LovTAP-VP16 system.
In each different cell line, we tested a transfection containing a light-sensitive protein and a target gene or readout. We chose to express easily detectable fluorescent proteins so that we could compare the efficiency of both systems in turning on a particular gene. The fluorescent protein gene could eventually be replaced by a gene coding for a medically-relevant protein of interest to the pharmaceutical industry.
Not Only Pharma
The use of our switch is not exclusively limited to the pharmaceutical industry. The LovTAP-VP16 switch can also be used in experimental biology to observe temporal gene expression suppression. Our project also has a multitude of other valuable applications, yet achieving them requires comprehension and consent of general public. That's why, in addition to our experimental work, we've presented our project and introduced the public to the field of synthetic biology, and attempted to engage in a two-sided dialog by polling different populations to get their opinion about synthetic biology.
Check the rest of our wiki to see our project more in detail!
 "
"