Team:CD-SCU-CHINA/Notebook
From 2012.igem.org
| (8 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
{{SCU-IGEM}} | {{SCU-IGEM}} | ||
| - | + | * '''Genome extraction''' | |
| - | + | ** Using gene extraction kit to isolate the whole genome of Alcanivorax borkumensis SK2<br> | |
| - | + | [[File:Genome 8.5.jpg]] | |
| - | + | * '''Gene isolate using PCR''' | |
| - | + | ** Normal primer design and '''gradient PCR''' to amplify the gene.<br> | |
| - | + | [[File:Gradent2.jpg]] | |
| - | + | * '''Site mutation''' | |
| + | ** Using the primer design by us, follow these tips: | ||
| + | 1,Full length not less than 28bp; | ||
| + | 2, 3'end to mutation site not less than 18bp; | ||
| + | 3, Mutation site to 5' end not less than 10bp. | ||
| + | * '''Gene purification ,Enzyme digest, ligation and transformation''' | ||
| + | In our experiment, we found the vector (we used) is hard to digest by two enzyme in the same time, it cost us a long time on the identification the correct plamid, unfortunately, it was frustrating. at last, we follow the advice from the teacher, degest one by one, and have an self-ligation test in order to have the correct digested vector. | ||
| + | * '''In-fusion cloning and tranformation''' | ||
| + | we use this method for the ligation of 6 genes, but follow the instrction of the Clontech company, we may waste a lot of money on it, so we search methods from the paper, first, we test four-way ligation, however, it turn out to be no colony. so we test three-ligation. it was useful. | ||
| + | Methods are as follows: | ||
| + | Vector: more than 50ng; | ||
| + | Gne inserts: more than 5:1 molar ratio to vector | ||
| + | In-fusion enzyme: 2ul every 10ul reaction system | ||
| + | ddH2O: Add water to 10ul<br> | ||
| - | + | [[File:Colony.jpg]] | |
| - | + | * '''Sequensing''' | |
| - | + | Done by company | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | * '''Colony PCR Plasmid extraction''' | |
| - | + | Colony PCR sometimes was not as convenient as we thougt. Sometimes plasmid extraction is better! | |
| - | + | [[File:Plasmid ex.jpg]] | |
| - | + | * '''Double Digestion for chek''' | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | * '''Temperament chromatography''' | |
| - | + | for the function test of the degradation of the two enzyme gene. | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
Latest revision as of 03:42, 27 September 2012
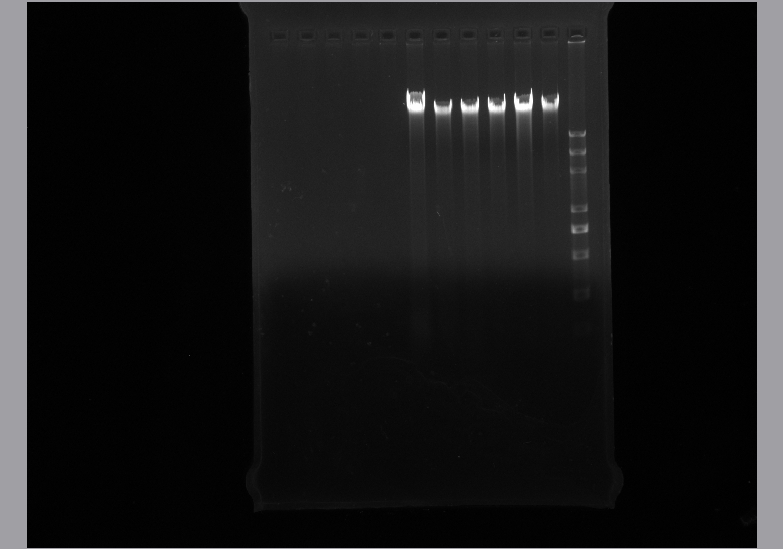
- Genome extraction
- Using gene extraction kit to isolate the whole genome of Alcanivorax borkumensis SK2
- Using gene extraction kit to isolate the whole genome of Alcanivorax borkumensis SK2
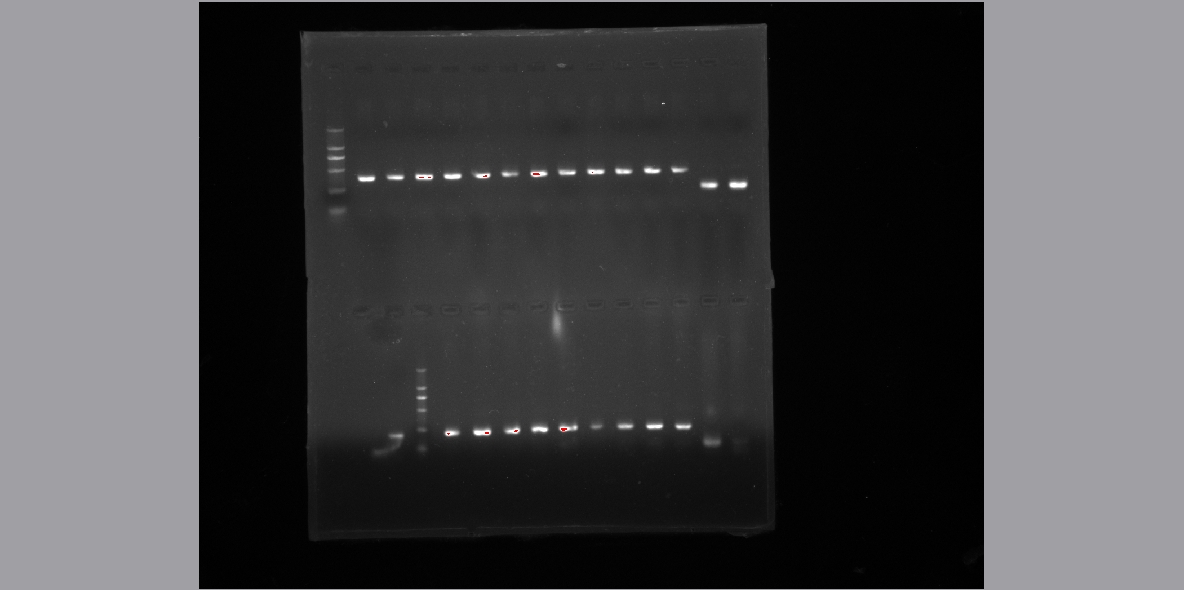
- Gene isolate using PCR
- Normal primer design and gradient PCR to amplify the gene.
- Normal primer design and gradient PCR to amplify the gene.
- Site mutation
- Using the primer design by us, follow these tips:
1,Full length not less than 28bp; 2, 3'end to mutation site not less than 18bp; 3, Mutation site to 5' end not less than 10bp.
- Gene purification ,Enzyme digest, ligation and transformation
In our experiment, we found the vector (we used) is hard to digest by two enzyme in the same time, it cost us a long time on the identification the correct plamid, unfortunately, it was frustrating. at last, we follow the advice from the teacher, degest one by one, and have an self-ligation test in order to have the correct digested vector.
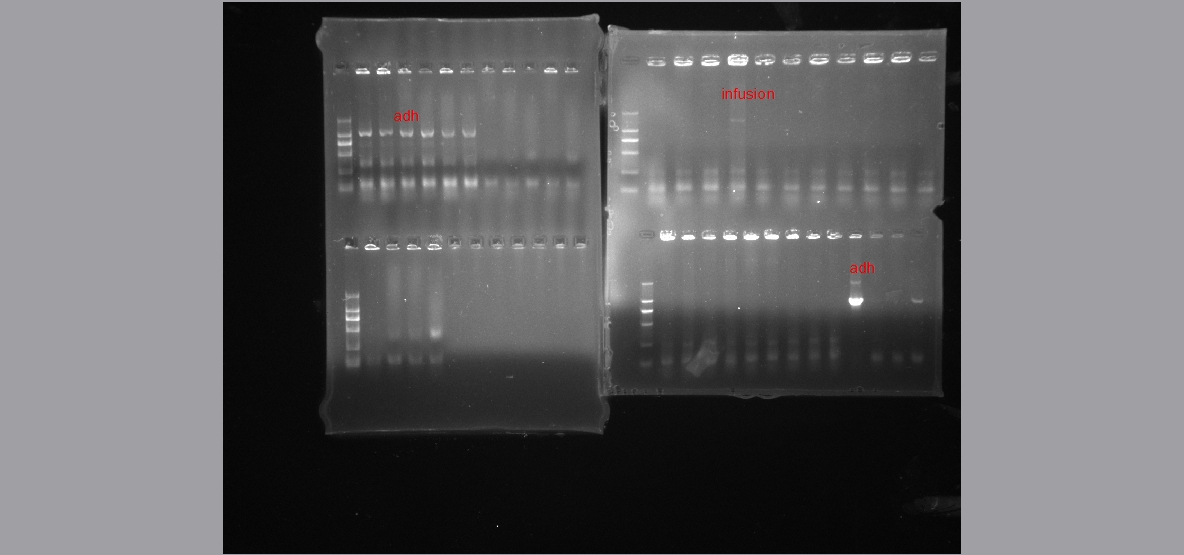
- In-fusion cloning and tranformation
we use this method for the ligation of 6 genes, but follow the instrction of the Clontech company, we may waste a lot of money on it, so we search methods from the paper, first, we test four-way ligation, however, it turn out to be no colony. so we test three-ligation. it was useful.
Methods are as follows:
Vector: more than 50ng;
Gne inserts: more than 5:1 molar ratio to vector
In-fusion enzyme: 2ul every 10ul reaction system
ddH2O: Add water to 10ul
- Sequensing
Done by company
- Colony PCR Plasmid extraction
Colony PCR sometimes was not as convenient as we thougt. Sometimes plasmid extraction is better!
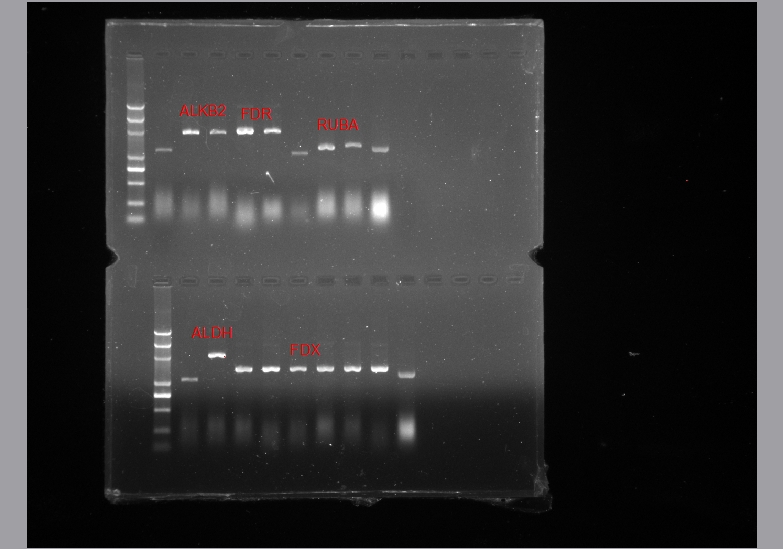
- Double Digestion for chek
- Temperament chromatography
for the function test of the degradation of the two enzyme gene.
 "
"