Team:TU Darmstadt/Project/Degradation
From 2012.igem.org
(→Degradation) |
(→Degradation) |
||
| (One intermediate revision not shown) | |||
| Line 49: | Line 49: | ||
For further characterisation the enzymes were overexpressed in ''E. coli'' strains [http://ecoliwiki.net/colipedia/index.php/TOP10 Top10], [http://ecoliwiki.net/colipedia/index.php/DH5_alpha DH5α], [http://ecoliwiki.net/colipedia/index.php/MG1655 Mg1655], screened on tributyrin agar and detected via flow cytometry after performing an antibody staining. The [https://2012.igem.org/Team:TU_Darmstadt/Project/Material_Science material science group] went even further and tried to examine them using AFM. | For further characterisation the enzymes were overexpressed in ''E. coli'' strains [http://ecoliwiki.net/colipedia/index.php/TOP10 Top10], [http://ecoliwiki.net/colipedia/index.php/DH5_alpha DH5α], [http://ecoliwiki.net/colipedia/index.php/MG1655 Mg1655], screened on tributyrin agar and detected via flow cytometry after performing an antibody staining. The [https://2012.igem.org/Team:TU_Darmstadt/Project/Material_Science material science group] went even further and tried to examine them using AFM. | ||
| + | |||
| + | We were able to control and reproduce the expression of our chimeric protein on the bacterial surface in three different ''E.coli'' strains, including the ethylene glycol metabolizing strain [http://ecoliwiki.net/colipedia/index.php/MG1655 Mg1655]. Furthermore we quantified the enzmyatic activity of our membrane bound fusion protein in vivo and the two PET-cleaving enzymes FsC/pNB-Est13 on selected substrates. | ||
The corresponding data is available in our [https://2012.igem.org/Team:TU_Darmstadt/Labjournal/Degradation labjournal]. If you want to know what happens with the PET after it is degradated to its TPA monomers continue with [https://2012.igem.org/Team:TU_Darmstadt/Project/Transport 2.Transport]. | The corresponding data is available in our [https://2012.igem.org/Team:TU_Darmstadt/Labjournal/Degradation labjournal]. If you want to know what happens with the PET after it is degradated to its TPA monomers continue with [https://2012.igem.org/Team:TU_Darmstadt/Project/Transport 2.Transport]. | ||
Latest revision as of 03:15, 27 September 2012
Degradation
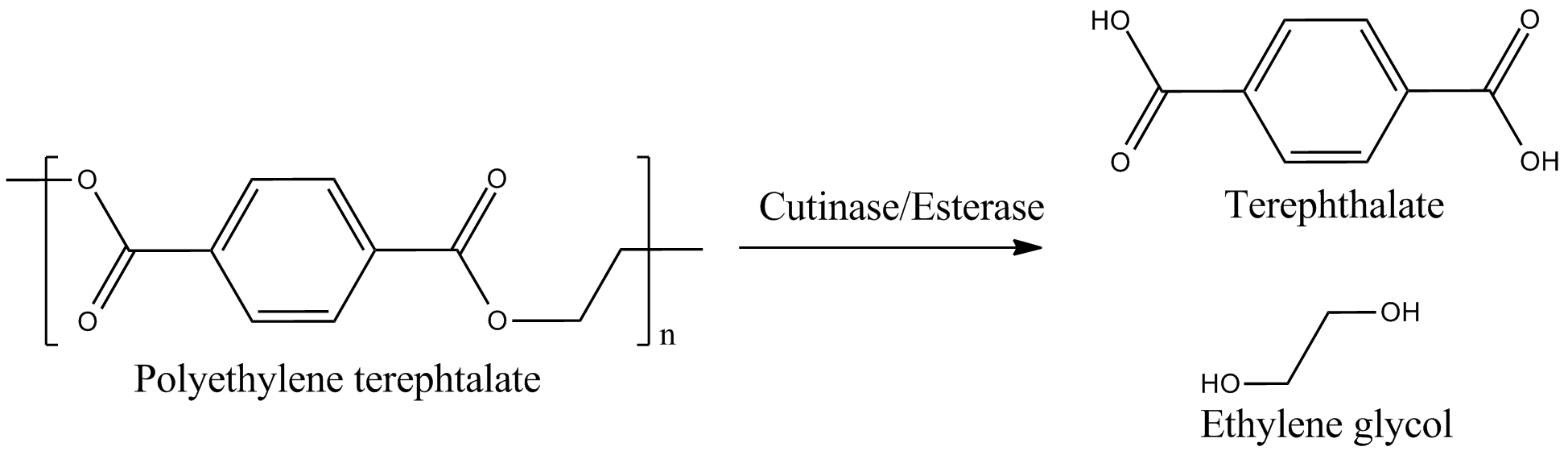
The degradation group consists of six undergraduates and two PhD student advisors. Our objective is the expression of a fusion protein on the surface of E. coli to enable a microbial polyethylenterephtalate (PET) degradation.
We identified three potential PET degradation enzymes from literature. Two of them are cutinases HiC (Humicola insolens cutinase) and FsC (Fusarium solani cutinase), the other namely pNB-Est13 beeing an esterase. After a short examination we dropped the HiC due to a temperature optimum of 80+°C. Shortly after the FsC was dropped as well, due to its toxicity for E.coli.In order to maximise the activity we decided to anchor and display the cutinase/esterase directly on the surface of the producing bacterial cell. Surface-exposed enzymes are directly accessible to the respective substrates which no longer have to traverse the cellular membrane barriers. Furthermore, the enzyme reaction occurs in a chemically more defined environment as compared to the interior of a microbial cell. We use the outer membrane proteins of Pseudomonas aeruginosa (EstA) as a membrane anchor and the signaling sequence of PhoA translocators to display the enzyme on the outer surface of E. coli cells. In addition the fusion protein contains a his-tag and a myc-tag for detection via flow cytometry after antibody staining or Western blot.
The signal sequence (PhoA), the catalytic domain (FsC/Est13) and EstA are assembled inline from their respective vectors. This is due to the fact, that by combining multiple parts in the standardized BioBrick vectors, scars with stop codons are generated that would effectively prevent the fusionproteins expression. In consideration of the DNA constructs length we pursued two PCR based synthesis strategies. One being the SKV (standard cloning procedure) the other being the SOE (standard overlap procedure). The first using primers and restriction sites for assembly the latter using overlapping primers. During both assembly procedures restriction sites of PstI, EcoRI, SpeI or XbaI were eliminated from the coding sequence by mutagene PCR. In the end we completely changed our assembly strategy, using synthesis products and BsaI sites to put our parts together. After completion the fusionprotein and its subunits were transfered to the BioBrick standard and sent to the registry.
For further characterisation the enzymes were overexpressed in E. coli strains [http://ecoliwiki.net/colipedia/index.php/TOP10 Top10], [http://ecoliwiki.net/colipedia/index.php/DH5_alpha DH5α], [http://ecoliwiki.net/colipedia/index.php/MG1655 Mg1655], screened on tributyrin agar and detected via flow cytometry after performing an antibody staining. The material science group went even further and tried to examine them using AFM.
We were able to control and reproduce the expression of our chimeric protein on the bacterial surface in three different E.coli strains, including the ethylene glycol metabolizing strain [http://ecoliwiki.net/colipedia/index.php/MG1655 Mg1655]. Furthermore we quantified the enzmyatic activity of our membrane bound fusion protein in vivo and the two PET-cleaving enzymes FsC/pNB-Est13 on selected substrates.
The corresponding data is available in our labjournal. If you want to know what happens with the PET after it is degradated to its TPA monomers continue with 2.Transport.
 "
"