Team:HokkaidoU Japan/Notebook/aggregation Week 8
From 2012.igem.org
| (15 intermediate revisions not shown) | |||
| Line 4: | Line 4: | ||
<!-- DO NOT EDIT OVER THIS LINE @iTakeshi --> | <!-- DO NOT EDIT OVER THIS LINE @iTakeshi --> | ||
<div class="hokkaidou-notebook-daily"> | <div class="hokkaidou-notebook-daily"> | ||
| - | ==August 20th== | + | ===August 20th=== |
| - | <div> | + | <div class="hokkaidou-section"> |
| - | ==Single colony isolation== | + | ====Single colony isolation==== |
| - | + | ||
Single colony isolation of pBAD-RBS-Ag43-dT on pSB1AK3. | Single colony isolation of pBAD-RBS-Ag43-dT on pSB1AK3. | ||
#Picked up one colony. | #Picked up one colony. | ||
| - | # | + | #Incubated on LBK (dt,RBS,T7) and LBC(pLacI-RBS-Ag43) for 20 hours. |
| - | + | ||
| - | ==Colony PCR== | + | ====Colony PCR==== |
| - | + | ||
Colony PCR to confirm that whether the pT7 and RBS was successfully ligated with pSB1C3 or not. | Colony PCR to confirm that whether the pT7 and RBS was successfully ligated with pSB1C3 or not. | ||
| - | |||
| - | |||
{|class="hokkaidou-table-pcr-reagent" | {|class="hokkaidou-table-pcr-reagent" | ||
|- | |- | ||
| Line 74: | Line 69: | ||
[[image:HokkaidoU2012 120820 pT7-RBS on pSB1C3 colop.jpg|thumb|Colony PCR result]] | [[image:HokkaidoU2012 120820 pT7-RBS on pSB1C3 colop.jpg|thumb|Colony PCR result]] | ||
| - | The results showed that ligated DNA has 300 ~ 400 bp and the desired products would have 331bp if it were amplified by 100bp up primer and 200bp down primer. Thus we confirmed that pT7-RBS on pSB1C3 was successfully ligated without no.6,9,10 colonies, but these 3 solution were | + | The results showed that ligated DNA has 300 ~ 400 bp and the desired products would have 331bp if it were amplified by 100bp up primer and 200bp down primer. Thus we confirmed that pT7-RBS on pSB1C3 was successfully ligated without no.6,9,10 colonies, but these 3 solution were evaporated because of our mistake. We selected No.4 and 5 colony for liquid culture. |
| - | + | ||
| - | + | ====PCR==== | |
| - | ==PCR== | + | |
| - | + | ||
PCR of BBa_I13453 (pBAD only part, it is not contain araC,). | PCR of BBa_I13453 (pBAD only part, it is not contain araC,). | ||
| - | |||
{|class="hokkaidou-table-pcr-reagent" | {|class="hokkaidou-table-pcr-reagent" | ||
|- | |- | ||
| Line 140: | Line 131: | ||
|} | |} | ||
Cycle:2~4 x 45 | Cycle:2~4 x 45 | ||
| - | |||
| - | |||
[[image:HokkaidoU2012_120821_pbad-pcr.jpg|thumb|PCR result]] | [[image:HokkaidoU2012_120821_pbad-pcr.jpg|thumb|PCR result]] | ||
| - | + | ====Liquid culture==== | |
| - | == | + | |
| - | + | ||
Liquid culture for 3 colonies of pBAD-RBS-Ag43-dT on pSB1AK3 (and pT7-RBS on pSB1C3 colony No.4 and 5 which were selected from the results of colony PCR). | Liquid culture for 3 colonies of pBAD-RBS-Ag43-dT on pSB1AK3 (and pT7-RBS on pSB1C3 colony No.4 and 5 which were selected from the results of colony PCR). | ||
#Added 2 ml of LBK (LBC) into culture tubes. | #Added 2 ml of LBK (LBC) into culture tubes. | ||
| - | #Resuspended 1 colonies | + | #Resuspended 1 colonies. |
#Incubated the tubes at 37C for 16 hours (19 hours). | #Incubated the tubes at 37C for 16 hours (19 hours). | ||
| - | |||
| + | <br style="line-height: 0; clear: both;" /> | ||
</div></div> | </div></div> | ||
| - | |||
<div class="hokkaidou-notebook-daily"> | <div class="hokkaidou-notebook-daily"> | ||
| - | ==August | + | ===August 21st=== |
| - | <div> | + | <div class="hokkaidou-section"> |
| - | ==PCR== | + | ====PCR==== |
| - | + | ||
PCR of pBAD(containing araC)-RBS. | PCR of pBAD(containing araC)-RBS. | ||
| - | And, we checked the plasmid which we | + | And, we checked the plasmid which we refined by plasmid extraction at August 18th is pBAD-RBS on pSB1A3 or not. |
{|class="hokkaidou-table-pcr-reagent" | {|class="hokkaidou-table-pcr-reagent" | ||
| Line 223: | Line 208: | ||
|} | |} | ||
Cycle:2~4 x 45 | Cycle:2~4 x 45 | ||
| - | |||
| - | |||
[[image:HokkaidoU2012_120821_pbad-rbs_pcr.jpg|thumb|PCR result]] | [[image:HokkaidoU2012_120821_pbad-rbs_pcr.jpg|thumb|PCR result]] | ||
| - | + | ====Aggregation check==== | |
| - | ==Aggregation check== | + | |
| - | + | ||
we cultured the E. coli, which transformed pBAD-RBS-Ag43-dT on pSB1AK3 in LBK. | we cultured the E. coli, which transformed pBAD-RBS-Ag43-dT on pSB1AK3 in LBK. | ||
We checked the construct by induction of L-arabinose after 16 hours incubate. | We checked the construct by induction of L-arabinose after 16 hours incubate. | ||
| - | |||
#2 ml of liquid culture divided two culture. (made two 1 ml culture) | #2 ml of liquid culture divided two culture. (made two 1 ml culture) | ||
| - | #Added 1 ml LBK in one culture as negative control. | + | #Added 1 ml LBK in one culture as a negative control. |
#Added 900 ul LBK and 100 ul 20% L-arabinose. | #Added 900 ul LBK and 100 ul 20% L-arabinose. | ||
| - | #Incubated at 37C 130 rpm for 2 | + | #Incubated at 37C 130 rpm for 2 hrs and 30 min. |
| - | #Placed tubes on the table at 30 | + | #Placed tubes on the table at 30 min. |
| - | + | ||
| - | == | + | ====Plasmid extraction==== |
| - | + | Mni-prep of pT7-RBS on pSB1C3 of colony No. 4 and 5 selected by the result of colony PCR at August 20th. We used Plasmid extraction kit of Nippon genetics: FastGene Plasmid mini kit and finally we eluted the DNA with 20 ul of elution buffer. | |
| - | Mni-prep of pT7-RBS on pSB1C3 of colony No. 4 and 5 selected by the result of colony PCR | + | [[image:HokkaidoU2012 120821 pT7-RBS on pSB1C3 ColonyNo. 4,5 mini-prep.jpg|thumb|Plasmid extraction result]] |
| + | The concentration of 20 ul of plasmid extraction products were too low to digestion or do something so we retry liquid culture of other number of colony solution: No. 1 and No. 2. | ||
| - | + | ====Liquid culture==== | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | == | + | |
| - | + | ||
Liquid culture for Ag43-dT on pSB1AK3 (and pT7-RBS on pSB1C3 colony No. 1 and 2 which were selected from the results of colony PCR). | Liquid culture for Ag43-dT on pSB1AK3 (and pT7-RBS on pSB1C3 colony No. 1 and 2 which were selected from the results of colony PCR). | ||
#Added 2 ml of LBA (LBC) into culture tubes. | #Added 2 ml of LBA (LBC) into culture tubes. | ||
| - | #Resuspended 2 colonies | + | #Resuspended 2 colonies. |
#Incubated the tubes at 37C for 18 hours (16 hours). | #Incubated the tubes at 37C for 18 hours (16 hours). | ||
| - | |||
| + | <br style="line-height: 0; clear: both;" /> | ||
</div></div> | </div></div> | ||
| - | |||
<div class="hokkaidou-notebook-daily"> | <div class="hokkaidou-notebook-daily"> | ||
| - | ==August | + | ===August 22nd=== |
| - | <div> | + | <div class="hokkaidou-section"> |
| - | == | + | ====Plasmid extraction==== |
| - | + | Mni-prep of pT7-RBS on pSB1C3 of colony No.1 and 2 selected by the result of colony PCR at August 20th. We used plasmid extraction kit of Nippon genetics: FastGene Plasmid mini kit and finally we eluted the DNA with 20 ul of elution buffer. | |
| - | Mni-prep of pT7-RBS on pSB1C3 of colony No.1 and 2 selected by the result of colony PCR | + | [[image:HokkaidoU2012 120822 pT7-RBS on pSB1C3 mini-prep No.jpg|thumb|plasmid extraction result]] |
| - | + | ||
| - | [[image:HokkaidoU2012 120822 pT7-RBS on pSB1C3 mini-prep No.jpg|thumb| | + | |
| - | + | ||
| - | + | ||
| - | One of Ag43-dT on pSB1AK3 culture did not get | + | One of Ag43-dT on pSB1AK3 culture did not get muddy. And another one is only a little muddy. |
| - | We tried | + | We tried plasmid extraction to the latter, we got the 20 ul of DNA solution. |
| - | And then, we did electrophoresis the | + | And then, we did electrophoresis the plasmid extraction products and (pBAD-RBS and pBAD) gel extract products. |
[[image:HokkaidoU2012_120822_take1.jpg|thumb|electrophoresis result(1% agarose gel)]] | [[image:HokkaidoU2012_120822_take1.jpg|thumb|electrophoresis result(1% agarose gel)]] | ||
[[image:HokkaidoU2012_120822_take2.jpg|thumb|electrophoresis result(2% agarose gel)]] | [[image:HokkaidoU2012_120822_take2.jpg|thumb|electrophoresis result(2% agarose gel)]] | ||
| - | |||
| - | ==PCR== | + | ====PCR==== |
| - | + | ||
PCR of pT7-RBS on pSB1C3.<br /> | PCR of pT7-RBS on pSB1C3.<br /> | ||
| - | |||
We used 4 kinds of primer set. | We used 4 kinds of primer set. | ||
<br /> | <br /> | ||
| Line 294: | Line 258: | ||
4 : 100b up , 200b down primer<br /> | 4 : 100b up , 200b down primer<br /> | ||
The density of primer solutions is 10 uM. | The density of primer solutions is 10 uM. | ||
| - | |||
{|class="hokkaidou-table-pcr-reagent" | {|class="hokkaidou-table-pcr-reagent" | ||
|- | |- | ||
| Line 324: | Line 287: | ||
|50 ul | |50 ul | ||
|} | |} | ||
| - | |||
{|class="hokkaidou-table-pcr-time" | {|class="hokkaidou-table-pcr-time" | ||
| Line 354: | Line 316: | ||
Cycle:2~4 x 45 | Cycle:2~4 x 45 | ||
| - | </ | + | <br style="line-height: 0; clear: both;" /> |
</div></div> | </div></div> | ||
| - | |||
<div class="hokkaidou-notebook-daily"> | <div class="hokkaidou-notebook-daily"> | ||
| - | ==August | + | ===August 23rd=== |
| - | <div> | + | <div class="hokkaidou-section"> |
| - | ==Digestion== | + | ====Digestion==== |
| - | + | ||
Digestion for making pBAD-RBS-Ag43-dT on pSB1AK3. | Digestion for making pBAD-RBS-Ag43-dT on pSB1AK3. | ||
| Line 429: | Line 389: | ||
|} | |} | ||
| - | + | ||
[[image:HokkaidoU2012_120824_ag43-dt-dig2.jpg|thumb|Ag43-dT digestion result]] | [[image:HokkaidoU2012_120824_ag43-dt-dig2.jpg|thumb|Ag43-dT digestion result]] | ||
[[image:HokkaidoU2012_120824_pbad-rbs-dig.jpg|thumb|pBAD-RBS digestion result]] | [[image:HokkaidoU2012_120824_pbad-rbs-dig.jpg|thumb|pBAD-RBS digestion result]] | ||
| - | ==Ethanol precipitation== | + | ====Ethanol precipitation==== |
| - | + | Ethanol precipitation to get more high concentration of Ag43-dT on pSB1AK3 solution digested by XbaI and SpeI. | |
| - | Ethanol precipitation to get more high concentration of Ag43-dT on pSB1AK3 solution | + | |
#Added 2 ul of NaoAc, 1.5 ul of glycogen and 50 ul of 100% ethanol. | #Added 2 ul of NaoAc, 1.5 ul of glycogen and 50 ul of 100% ethanol. | ||
| - | #Centrifuged | + | #Centrifuged at 15000 rpm, 10 min at 4C. |
| - | # | + | #Removed supernatant and added 220 ul of 70% ethanol. |
| - | #Centrifuged | + | #Centrifuged at 15000 rpm, 10 min at 4C. |
| - | # | + | #Removed supernatant and dried out at room temperature, after that added 10 ul of DW. |
| - | + | ====Digestion==== | |
| - | ==Digestion== | + | Digestion to divide Ag43-dT and pSB1AK3 which has same number of bp as Ag43-dT by digested by HindIII. |
| - | + | ||
| - | Digestion to divide Ag43-dT and pSB1AK3 which has same number of bp as Ag43-dT by | + | |
{|class="hokkaidou-table-digestion" | {|class="hokkaidou-table-digestion" | ||
|- | |- | ||
| Line 451: | Line 408: | ||
|9 ul | |9 ul | ||
|- | |- | ||
| - | |HindIII( | + | |HindIII(15 U/ul) |
|1 ul | |1 ul | ||
|- | |- | ||
| Line 486: | Line 443: | ||
[[image:HokkaidoU2012 120824 digestion HindIII Ag43-dT with Ag43.jpg|thumb|digestion result]] | [[image:HokkaidoU2012 120824 digestion HindIII Ag43-dT with Ag43.jpg|thumb|digestion result]] | ||
| + | From this result, we confirmed that the pSB1AK3 was successfully digested by HindIII, but it could not confirm how many pSB1AK3 were remained as non-digested products. | ||
| + | ====Gel extraction==== | ||
| + | Gel extraction for digestion product. We used FastGene Gel&PCR extraction kit(NipponGenetics)and got 50 ul of DNA solution. | ||
| - | + | <br style="line-height: 0; clear: both;" /> | |
| - | + | ||
| - | < | + | |
| - | = | + | |
| - | + | ||
| - | + | ||
| - | + | ||
</div></div> | </div></div> | ||
| - | |||
<div class="hokkaidou-notebook-daily"> | <div class="hokkaidou-notebook-daily"> | ||
| - | ==August 24th== | + | ===August 24th=== |
| - | <div> | + | <div class="hokkaidou-section"> |
| - | ==Digestion== | + | ====Digestion==== |
| - | + | ||
| - | + | ||
| - | + | ||
| + | Digested pT7-RBS on pSB1C3 by SpeI, and Ag43-dT on pSB1AK3 digested by EcoRI and XbaI, pBAD-RBS digested by EcoRI and PstI. | ||
| + | <br /> | ||
| + | <br /> | ||
Ag43-dT on pSB1AK3 | Ag43-dT on pSB1AK3 | ||
| - | + | <br /><br /> | |
| - | + | EcoRI/XbaI | |
| - | + | ||
| - | + | ||
{|class="hokkaidou-table-digestion" | {|class="hokkaidou-table-digestion" | ||
|- | |- | ||
| Line 520: | Line 471: | ||
|- | |- | ||
|XbaI | |XbaI | ||
| - | |1 | + | |1 ul |
|- | |- | ||
|10xM buffer | |10xM buffer | ||
| Line 533: | Line 484: | ||
| - | + | EcoRI | |
| - | + | ||
{|class="hokkaidou-table-digestion" | {|class="hokkaidou-table-digestion" | ||
|- | |- | ||
| Line 554: | Line 504: | ||
| - | + | XbaI | |
| - | + | ||
{|class="hokkaidou-table-digestion" | {|class="hokkaidou-table-digestion" | ||
|- | |- | ||
| Line 562: | Line 511: | ||
|- | |- | ||
|XbaI | |XbaI | ||
| - | |1 | + | |1 ul |
|- | |- | ||
|10xM buffer | |10xM buffer | ||
| Line 575: | Line 524: | ||
| - | + | pBAD-RBS<br /> | |
| - | pBAD-RBS | + | EcoRI/PstI |
{|class="hokkaidou-table-digestion" | {|class="hokkaidou-table-digestion" | ||
|- | |- | ||
| - | |DNA solution ( | + | |DNA solution (100 ng/ul) |
|12 ul | |12 ul | ||
|- | |- | ||
| Line 586: | Line 535: | ||
|- | |- | ||
|SpeI | |SpeI | ||
| - | |1 | + | |1 ul |
|- | |- | ||
|10xH buffer | |10xH buffer | ||
| Line 597: | Line 546: | ||
|20 ul | |20 ul | ||
|} | |} | ||
| - | |||
| Line 618: | Line 566: | ||
|20 ul | |20 ul | ||
|} | |} | ||
| - | |||
| Line 639: | Line 586: | ||
|HOLD | |HOLD | ||
|} | |} | ||
| - | |||
| Line 645: | Line 591: | ||
About pT7-RBS on pSB1C3, we successfully digested the plasmid DNA and converted it to linear DNA. | About pT7-RBS on pSB1C3, we successfully digested the plasmid DNA and converted it to linear DNA. | ||
| - | + | ====Gel extraction==== | |
| - | ==Gel extraction== | + | |
| - | + | ||
Gel extraction for digestion product. We used FastGene Gel&PCR extraction kit(NipponGenetics)and got 50 ul of DNA solution. | Gel extraction for digestion product. We used FastGene Gel&PCR extraction kit(NipponGenetics)and got 50 ul of DNA solution. | ||
| - | + | ====Ethanol Precipitation==== | |
| - | + | ||
| - | ==Ethanol Precipitation== | + | |
| - | + | ||
Ethanol precipitation for pT7-RBS on pSB1C3 and Ag43-dT digestion products. | Ethanol precipitation for pT7-RBS on pSB1C3 and Ag43-dT digestion products. | ||
#Added 5 ul of NaoAc, 1.5 ul of glycogen and 125 ul of 100% ethanol. | #Added 5 ul of NaoAc, 1.5 ul of glycogen and 125 ul of 100% ethanol. | ||
| - | #Centrifuged | + | #Centrifuged at 15000 rpm, 10 min at 4C. |
| - | # | + | #Removed supernatant and added 220 ul of 70% ethanol. |
| - | #Centrifuged | + | #Centrifuged at 15000 rpm, 10 min at 4C. |
| - | # | + | #Removed supernatant and dried out at room temperature, after that added 10 ul of DW. |
| - | + | ||
| - | ==Ligation== | + | ====Ligation==== |
| - | + | ||
Ligation for Ag43-dT as insert and pT7-RBS on pSB1C3 as vector. | Ligation for Ag43-dT as insert and pT7-RBS on pSB1C3 as vector. | ||
We used Ligation Mighty Mix (TAKARA BIO INC.) which contains ligase and buffer. | We used Ligation Mighty Mix (TAKARA BIO INC.) which contains ligase and buffer. | ||
| - | |||
{|class="hokkaidou-table-ligation" | {|class="hokkaidou-table-ligation" | ||
| Line 706: | Line 644: | ||
[[image:HokkaidoU2012 120825 Ligation pT7-RBS-Ag43-dT on psB1C3.jpg|thumb|Ligation result]] | [[image:HokkaidoU2012 120825 Ligation pT7-RBS-Ag43-dT on psB1C3.jpg|thumb|Ligation result]] | ||
| - | |||
| - | |||
| + | <br style="line-height: 0; clear: both;" /> | ||
</div></div> | </div></div> | ||
| - | |||
<div class="hokkaidou-notebook-daily"> | <div class="hokkaidou-notebook-daily"> | ||
| - | ==August 25th== | + | ===August 25th=== |
| - | <div> | + | <div class="hokkaidou-section"> |
| - | ==Transformation== | + | ====Transformation==== |
| - | + | Transformation for ligation product into DH5α. | |
| - | Transformation for ligation product | + | |
#Added 2 ul of DNA to 50 ul of thawed competent cells on ice. | #Added 2 ul of DNA to 50 ul of thawed competent cells on ice. | ||
#Incubated on ice for 30 min. | #Incubated on ice for 30 min. | ||
| Line 727: | Line 662: | ||
#Added 450 ul of LB to 50 ul of the culture and plated 300 ul of it onto second dish and spread. | #Added 450 ul of LB to 50 ul of the culture and plated 300 ul of it onto second dish and spread. | ||
#Incubated the plates at 37C for hours. | #Incubated the plates at 37C for hours. | ||
| - | |||
| - | ==Colony PCR== | + | ====Colony PCR==== |
| - | + | ||
Colony PCR to confirm that whether the pT7-RBS-Ag43-dT on pSB1C3 was successfully ligated or not. | Colony PCR to confirm that whether the pT7-RBS-Ag43-dT on pSB1C3 was successfully ligated or not. | ||
| - | |||
{|class="hokkaidou-table-pcr-reagent" | {|class="hokkaidou-table-pcr-reagent" | ||
| Line 790: | Line 722: | ||
[[image:HokkaidoU2012 120825 coloP pT7-RBS-Ag43-dT on pSB1C3.jpg|thumb|Colony PCR result]] | [[image:HokkaidoU2012 120825 coloP pT7-RBS-Ag43-dT on pSB1C3.jpg|thumb|Colony PCR result]] | ||
| - | The results showed that desired DNA were not existed in these picked up colonies. We observed our ethanol precipitation and | + | The results showed that desired DNA were not existed in these picked up colonies. We observed our ethanol precipitation and electrophoresis result image of ligation products, then noticed that the concentration of each ethanol precipitation product was reversed. This means vector DNA would be self-ligated by the high ratio of molar in ligated DNA solution. We decided to try the DNA synthesis from digestion reaction of vector DNA once more time. |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| + | ====Digestion==== | ||
| + | Digest vector DNA: pT7-RBS on pSB1C3 by SpeI. Not to leave the plasmid DNA as plasmid DNA, we digested the DNA for 18 hrs. | ||
pT7-RBS on pSB1C3 (SpeI) | pT7-RBS on pSB1C3 (SpeI) | ||
{|class="hokkaidou-table-digestion" | {|class="hokkaidou-table-digestion" | ||
|- | |- | ||
| - | |DNA solution ( | + | |DNA solution (20 ng/ul) |
|4 ul | |4 ul | ||
|- | |- | ||
| Line 835: | Line 764: | ||
|HOLD | |HOLD | ||
|} | |} | ||
| - | </ | + | |
| + | <br style="line-height: 0; clear: both;" /> | ||
</div></div> | </div></div> | ||
| - | |||
<div class="hokkaidou-notebook-daily"> | <div class="hokkaidou-notebook-daily"> | ||
| - | ==August 26th== | + | ===August 26th=== |
| - | <div | + | <div class="hokkaidou-section"> |
| - | + | ||
| - | = | + | |
| - | + | ||
| - | + | ||
| + | ======Ligation====== | ||
| + | Ligation of pBAD-RBS and Ag43-dT on pSB1AK3 | ||
{|class="hokkaidou-table-ligation" | {|class="hokkaidou-table-ligation" | ||
|- | |- | ||
| Line 881: | Line 808: | ||
|Hold | |Hold | ||
|} | |} | ||
| - | |||
| - | ===Transformation=== | + | ======Transformation====== |
| - | + | Transformation for ligation product into DH5α. | |
| - | Transformation for ligation product | + | |
#Added 2 ul of DNA to 50 ul of thawed competent cells on ice. | #Added 2 ul of DNA to 50 ul of thawed competent cells on ice. | ||
#Incubated on ice for 30 min. | #Incubated on ice for 30 min. | ||
| Line 892: | Line 817: | ||
#Added 450 ul of LB to 50 ul of the culture and plated 300 ul of it onto second LBA dish and spread. | #Added 450 ul of LB to 50 ul of the culture and plated 300 ul of it onto second LBA dish and spread. | ||
#Incubated the plates at 37C for 17 hrs and 30 min. | #Incubated the plates at 37C for 17 hrs and 30 min. | ||
| - | |||
| - | + | ====Gel extraction==== | |
| - | ==Gel extraction== | + | |
| - | + | ||
Gel extraction for digestion product. We used FastGene Gel&PCR extraction kit(NipponGenetics)and got 50 ul of DNA solution. | Gel extraction for digestion product. We used FastGene Gel&PCR extraction kit(NipponGenetics)and got 50 ul of DNA solution. | ||
| - | + | ====Ethanol Precipitation==== | |
| - | + | ||
| - | ==Ethanol Precipitation== | + | |
| - | + | ||
Ethanol precipitation for pT7-RBS on pSB1C3 and Ag43-dT digestion products. | Ethanol precipitation for pT7-RBS on pSB1C3 and Ag43-dT digestion products. | ||
#Added 5 ul of NaoAc, 1.5 ul of glycogen and 125 ul of 100% ethanol. | #Added 5 ul of NaoAc, 1.5 ul of glycogen and 125 ul of 100% ethanol. | ||
| - | #Centrifuged | + | #Centrifuged at 14000 rpm, 30 min at 4C. |
| - | # | + | #Removed supernatant and added 220 ul of 70% ethanol. |
| - | #Centrifuged | + | #Centrifuged at 15000 rpm, 15 min at 4C. |
| - | # | + | #Removed supernatant and dried out at room temperature, after that added 10 ul of DW. |
| - | + | ||
[[image:HokkaidoU2012 120826 Ethanol precipitation Ag43-dT and pT7-RBS on pSB1C3 d+.jpg|thumb|Ethanol precipitation result]] | [[image:HokkaidoU2012 120826 Ethanol precipitation Ag43-dT and pT7-RBS on pSB1C3 d+.jpg|thumb|Ethanol precipitation result]] | ||
| - | |||
| - | ==Ligation== | + | ====Ligation==== |
| - | + | ||
Ligation for Ag43-dT as insert and pT7-RBS on pSB1C3 as vector. | Ligation for Ag43-dT as insert and pT7-RBS on pSB1C3 as vector. | ||
We use Ligation Mighty Mix (TAKARA BIO INC.) which contains ligase and buffer. | We use Ligation Mighty Mix (TAKARA BIO INC.) which contains ligase and buffer. | ||
| - | |||
{|class="hokkaidou-table-ligation" | {|class="hokkaidou-table-ligation" | ||
| Line 956: | Line 871: | ||
|} | |} | ||
| - | + | ====Transformation==== | |
| - | + | Transformation for ligation product into DH5α. | |
| - | + | ||
| - | ==Transformation== | + | |
| - | + | ||
| - | Transformation for ligation product | + | |
#Added 2 ul of DNA to 50 ul of thawed competent cells on ice. | #Added 2 ul of DNA to 50 ul of thawed competent cells on ice. | ||
#Incubated on ice for 30 min. | #Incubated on ice for 30 min. | ||
| Line 969: | Line 880: | ||
#Plated 300 ul of the culture onto first dish and spread. | #Plated 300 ul of the culture onto first dish and spread. | ||
#Added 450 ul of LB to 50 ul of the culture and plated 300 ul of it onto second dish and spread. | #Added 450 ul of LB to 50 ul of the culture and plated 300 ul of it onto second dish and spread. | ||
| - | #Incubated the plates at 37C for | + | #Incubated the plates at 37C for 18 hrs. |
| - | </ | + | <br style="line-height: 0; clear: both;" /> |
</div></div> | </div></div> | ||
Latest revision as of 03:10, 27 September 2012
Contents |
August 20th
Single colony isolation
Single colony isolation of pBAD-RBS-Ag43-dT on pSB1AK3.
- Picked up one colony.
- Incubated on LBK (dt,RBS,T7) and LBC(pLacI-RBS-Ag43) for 20 hours.
Colony PCR
Colony PCR to confirm that whether the pT7 and RBS was successfully ligated with pSB1C3 or not.
| DNA solution | 4 ul |
| Kapa-Taq(Taq polymerase) | 5 ul |
| Forward Primer(100bp up primer) | 0.5 ul |
| Reverse Primer(200bp down primer) | 0.5 ul |
| Total | 10 ul |
| Number | Degree | Second |
| 1 | 95 | 120 |
| 2 | 95 | 30 |
| 3 | 53.2 | 30 |
| 4 | 72 | 60 |
| 5 | 72 | 60 |
| 6 | 4 | HOLD |
Cycle:2~4 x 35
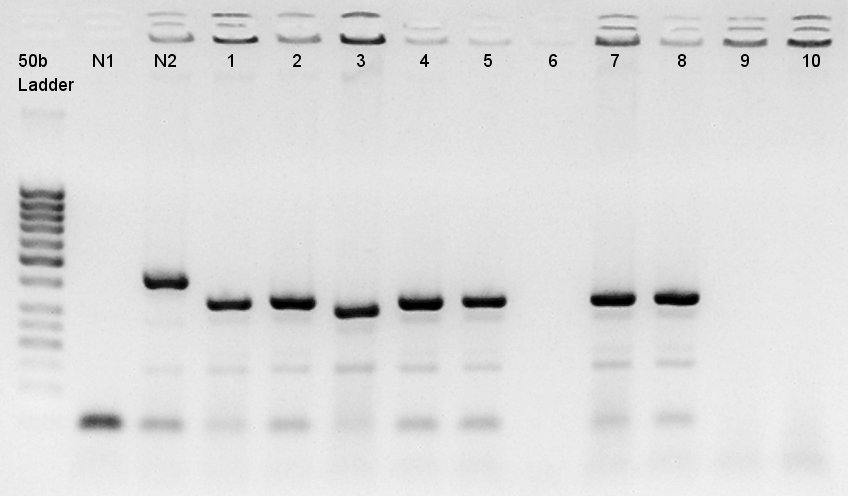
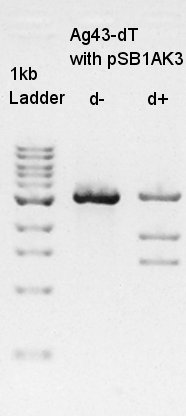
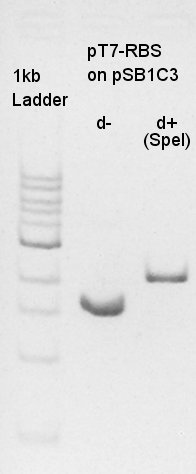
We used N1 (DW only) and N2 (pBAD(containing araC)-RBS on pSB1A3) as controls. Desired product is about 300~400bp.
The results showed that ligated DNA has 300 ~ 400 bp and the desired products would have 331bp if it were amplified by 100bp up primer and 200bp down primer. Thus we confirmed that pT7-RBS on pSB1C3 was successfully ligated without no.6,9,10 colonies, but these 3 solution were evaporated because of our mistake. We selected No.4 and 5 colony for liquid culture.
PCR
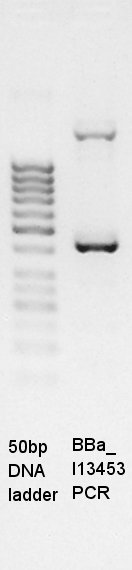
PCR of BBa_I13453 (pBAD only part, it is not contain araC,).
| DNA solution | 1 ul |
| KOD-Plus-NEO(Taq polymerase) | 1 ul |
| dNTP | 5 ul |
| MgSO4 | 3 ul |
| KOD-Plus-NEO Buffer | 5 ul |
| Forward Primer(Ag43-f4 primer: 10 uM) | 1 ul |
| Reverse Primer(PS-R primer: 10 uM) | 1 ul |
| DW | 33 ul |
| Total | 50 ul |
| Number | Degree | Second |
| 1 | 94 | 120 |
| 2 | 98 | 10 |
| 3 | 58 | 30 |
| 4 | 68 | 30 |
| 5 | 4 | HOLD |
Cycle:2~4 x 45
Liquid culture
Liquid culture for 3 colonies of pBAD-RBS-Ag43-dT on pSB1AK3 (and pT7-RBS on pSB1C3 colony No.4 and 5 which were selected from the results of colony PCR).
- Added 2 ml of LBK (LBC) into culture tubes.
- Resuspended 1 colonies.
- Incubated the tubes at 37C for 16 hours (19 hours).
August 21st
PCR
PCR of pBAD(containing araC)-RBS. And, we checked the plasmid which we refined by plasmid extraction at August 18th is pBAD-RBS on pSB1A3 or not.
| DNA solution | 1 ul |
| KOD-Plus-NEO(Taq polymerase) | 1 ul |
| dNTP | 5 ul |
| MgSO4 | 3 ul |
| KOD-Plus-NEO Buffer | 5 ul |
| Forward Primer(Ag43-f4 primer: 10 uM) | 1 ul |
| Reverse Primer(PS-R primer: 10 uM) | 1 ul |
| DW | 33 ul |
| Total | 50 ul |
| Number | Degree | Second |
| 1 | 94 | 120 |
| 2 | 98 | 10 |
| 3 | 58 | 30 |
| 4 | 68 | 30 |
| 5 | 4 | HOLD |
Cycle:2~4 x 45
Aggregation check
we cultured the E. coli, which transformed pBAD-RBS-Ag43-dT on pSB1AK3 in LBK. We checked the construct by induction of L-arabinose after 16 hours incubate.
- 2 ml of liquid culture divided two culture. (made two 1 ml culture)
- Added 1 ml LBK in one culture as a negative control.
- Added 900 ul LBK and 100 ul 20% L-arabinose.
- Incubated at 37C 130 rpm for 2 hrs and 30 min.
- Placed tubes on the table at 30 min.
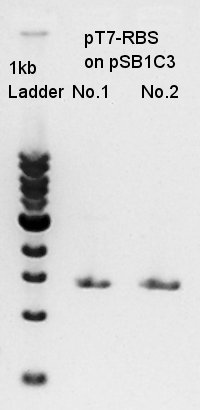
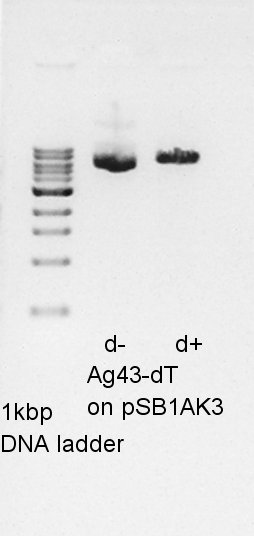
Plasmid extraction
Mni-prep of pT7-RBS on pSB1C3 of colony No. 4 and 5 selected by the result of colony PCR at August 20th. We used Plasmid extraction kit of Nippon genetics: FastGene Plasmid mini kit and finally we eluted the DNA with 20 ul of elution buffer.
The concentration of 20 ul of plasmid extraction products were too low to digestion or do something so we retry liquid culture of other number of colony solution: No. 1 and No. 2.
Liquid culture
Liquid culture for Ag43-dT on pSB1AK3 (and pT7-RBS on pSB1C3 colony No. 1 and 2 which were selected from the results of colony PCR).
- Added 2 ml of LBA (LBC) into culture tubes.
- Resuspended 2 colonies.
- Incubated the tubes at 37C for 18 hours (16 hours).
August 22nd
Plasmid extraction
Mni-prep of pT7-RBS on pSB1C3 of colony No.1 and 2 selected by the result of colony PCR at August 20th. We used plasmid extraction kit of Nippon genetics: FastGene Plasmid mini kit and finally we eluted the DNA with 20 ul of elution buffer.
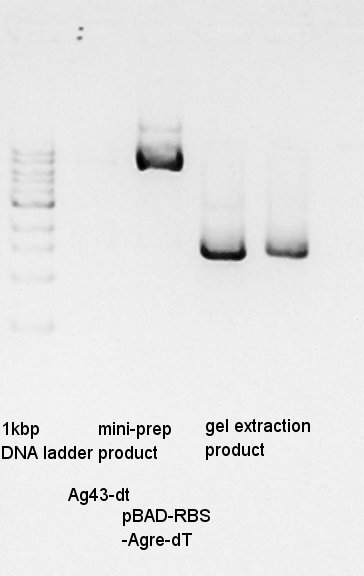
One of Ag43-dT on pSB1AK3 culture did not get muddy. And another one is only a little muddy. We tried plasmid extraction to the latter, we got the 20 ul of DNA solution. And then, we did electrophoresis the plasmid extraction products and (pBAD-RBS and pBAD) gel extract products.
PCR
PCR of pT7-RBS on pSB1C3.
We used 4 kinds of primer set.
1 : EX-F , PS-R primer
2 : EX-F , 200b down primer
3 : 100b up , PS-R primer
4 : 100b up , 200b down primer
The density of primer solutions is 10 uM.
| DNA solution | 1 ul |
| KOD-Plus-NEO(Taq polymerase) | 1 ul |
| dNTP | 5 ul |
| MgSO4 | 3 ul |
| KOD-Plus-NEO Buffer | 5 ul |
| Forward Primer | 1 ul |
| Reverse Primer | 1 ul |
| DW | 33 ul |
| Total | 50 ul |
| Number | Degree | Second |
| 1 | 94 | 120 |
| 2 | 98 | 10 |
| 3 | 58 | 30 |
| 4 | 68 | 30 |
| 5 | 4 | HOLD |
Cycle:2~4 x 45
August 23rd
Digestion
Digestion for making pBAD-RBS-Ag43-dT on pSB1AK3.
Digestion of pBAD-RBS.
| pBAD-RBS (100 ng/ul) | 12 ul |
| Eco RI | 1 ul |
| SpeI | 1 ul |
| 10xH buffer | 2 ul |
| DW | 4 ul |
| Total | 20 ul |
Digestion of Ag43-dT on pSB1AK3.
| Ag43-dT (120 ng/ul) | 7 ul |
| Eco RI | 1 ul |
| XbaI | 1 ul |
| 10xM buffer | 2 ul |
| DW | 9 ul |
| Total | 20 ul |
| Number | Degree | Minute |
| 1 | 37 | 180 |
| 2 | 60 | 15 |
| 3 | 4 | HOLD |
Ethanol precipitation
Ethanol precipitation to get more high concentration of Ag43-dT on pSB1AK3 solution digested by XbaI and SpeI.
- Added 2 ul of NaoAc, 1.5 ul of glycogen and 50 ul of 100% ethanol.
- Centrifuged at 15000 rpm, 10 min at 4C.
- Removed supernatant and added 220 ul of 70% ethanol.
- Centrifuged at 15000 rpm, 10 min at 4C.
- Removed supernatant and dried out at room temperature, after that added 10 ul of DW.
Digestion
Digestion to divide Ag43-dT and pSB1AK3 which has same number of bp as Ag43-dT by digested by HindIII.
| DNA solution ( 257ng/ul) | 9 ul |
| HindIII(15 U/ul) | 1 ul |
| 10xM buffer | 2 ul |
| DW | 8 ul |
| Total | 20 ul |
| Number | Degree | Minute |
| 1 | 37 | 180 |
| 2 | 70 | 15 |
| 3 | 4 | HOLD |
From this result, we confirmed that the pSB1AK3 was successfully digested by HindIII, but it could not confirm how many pSB1AK3 were remained as non-digested products.
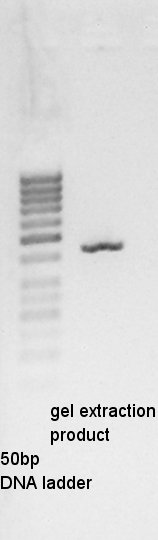
Gel extraction
Gel extraction for digestion product. We used FastGene Gel&PCR extraction kit(NipponGenetics)and got 50 ul of DNA solution.
August 24th
Digestion
Digested pT7-RBS on pSB1C3 by SpeI, and Ag43-dT on pSB1AK3 digested by EcoRI and XbaI, pBAD-RBS digested by EcoRI and PstI.
Ag43-dT on pSB1AK3
EcoRI/XbaI
| DNA solution ( 120ng/ul) | 7 ul |
| EcoRI | 1 ul |
| XbaI | 1 ul |
| 10xM buffer | 2 ul |
| DW | 9 ul |
| Total | 20 ul |
EcoRI
| DNA solution ( 120ng/ul) | 7 ul |
| EcoRI | 1 ul |
| 10xM buffer | 2 ul |
| DW | 10 ul |
| Total | 20 ul |
XbaI
| DNA solution ( 120ng/ul) | 7 ul |
| XbaI | 1 ul |
| 10xM buffer | 2 ul |
| DW | 10 ul |
| Total | 20 ul |
pBAD-RBS
EcoRI/PstI
| DNA solution (100 ng/ul) | 12 ul |
| EcoRI | 1 ul |
| SpeI | 1 ul |
| 10xH buffer | 2 ul |
| DW | 4 ul |
| Total | 20 ul |
pT7-RBS on pSB1C3 (SpeI)
| DNA solution ( 20ng/ul) | 4 ul |
| SpeI | 1 ul |
| 10xM buffer | 2 ul |
| DW | 13 ul |
| Total | 20 ul |
| Number | Degree | Minute |
| 1 | 37 | 180 |
| 2 | 60 | 15 |
| 3 | 4 | HOLD |
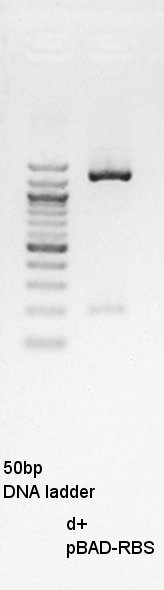
About pT7-RBS on pSB1C3, we successfully digested the plasmid DNA and converted it to linear DNA.
Gel extraction
Gel extraction for digestion product. We used FastGene Gel&PCR extraction kit(NipponGenetics)and got 50 ul of DNA solution.
Ethanol Precipitation
Ethanol precipitation for pT7-RBS on pSB1C3 and Ag43-dT digestion products.
- Added 5 ul of NaoAc, 1.5 ul of glycogen and 125 ul of 100% ethanol.
- Centrifuged at 15000 rpm, 10 min at 4C.
- Removed supernatant and added 220 ul of 70% ethanol.
- Centrifuged at 15000 rpm, 10 min at 4C.
- Removed supernatant and dried out at room temperature, after that added 10 ul of DW.
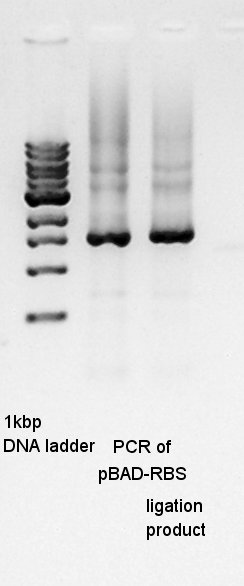
Ligation
Ligation for Ag43-dT as insert and pT7-RBS on pSB1C3 as vector. We used Ligation Mighty Mix (TAKARA BIO INC.) which contains ligase and buffer.
| Vector DNA | 4 ul |
| Insert DNA | 4 ul |
| DW | 2 ul |
| Ligation Mighty Mix | 10 ul |
| Total | 20 ul |
Ligation reaction time was in detail below.
| Degree | Minute |
| 16 | 30 |
| 65 | 10 |
| 4 | Hold |
August 25th
Transformation
Transformation for ligation product into DH5α.
- Added 2 ul of DNA to 50 ul of thawed competent cells on ice.
- Incubated on ice for 30 min.
- Added 350 ul of LB.
- Incubated the cells for 2 hours at 37C.
- Prepared and Labeled two plastic plates with LBC.
- Plated 300 ul of the culture onto first dish and spread.
- Added 450 ul of LB to 50 ul of the culture and plated 300 ul of it onto second dish and spread.
- Incubated the plates at 37C for hours.
Colony PCR
Colony PCR to confirm that whether the pT7-RBS-Ag43-dT on pSB1C3 was successfully ligated or not.
| DNA solution | 4 ul |
| Kapa-Taq(Taq polymerase) | 5 ul |
| Forward Primer(ag43-f4 primer) | 0.5 ul |
| Reverse Primer(200bp down primer) | 0.5 ul |
| Total | 10 ul |
| Number | Degree | Second |
| 1 | 95 | 120 |
| 2 | 95 | 30 |
| 3 | 53.0 | 30 |
| 4 | 72 | 60 |
| 5 | 72 | 60 |
| 6 | 4 | HOLD |
Cycle:2~4 x 35
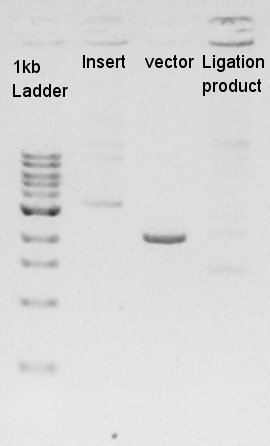
We used N1 (DW only) and N2 (Ag43-dT on pSB1AK3) as controls. Desired product is about 695bp.
The results showed that desired DNA were not existed in these picked up colonies. We observed our ethanol precipitation and electrophoresis result image of ligation products, then noticed that the concentration of each ethanol precipitation product was reversed. This means vector DNA would be self-ligated by the high ratio of molar in ligated DNA solution. We decided to try the DNA synthesis from digestion reaction of vector DNA once more time.
Digestion
Digest vector DNA: pT7-RBS on pSB1C3 by SpeI. Not to leave the plasmid DNA as plasmid DNA, we digested the DNA for 18 hrs. pT7-RBS on pSB1C3 (SpeI)
| DNA solution (20 ng/ul) | 4 ul |
| SpeI | 1 ul |
| 10xM buffer | 2 ul |
| DW | 13 ul |
| Total | 20 ul |
| Number | Degree | Minute |
| 1 | 37 | 600 (10 hours) |
| 2 | 60 | 15 |
| 3 | 4 | HOLD |
August 26th
Ligation
Ligation of pBAD-RBS and Ag43-dT on pSB1AK3
| pBAD-RBS | 3 ul |
| Ag43-dT on pSB1AK3 | 1 ul |
| Ligation Mighty Mix(TAKARA) | 5 ul |
| DW | 1 ul |
| Total | 10 ul |
| Degree | Minute |
| 16 | 30 |
| 65 | 10 |
| 4 | Hold |
Transformation
Transformation for ligation product into DH5α.
- Added 2 ul of DNA to 50 ul of thawed competent cells on ice.
- Incubated on ice for 30 min.
- Added 350 ul of LB.
- Plated 300 ul of the culture onto first LBA dish and spread.
- Added 450 ul of LB to 50 ul of the culture and plated 300 ul of it onto second LBA dish and spread.
- Incubated the plates at 37C for 17 hrs and 30 min.
Gel extraction
Gel extraction for digestion product. We used FastGene Gel&PCR extraction kit(NipponGenetics)and got 50 ul of DNA solution.
Ethanol Precipitation
Ethanol precipitation for pT7-RBS on pSB1C3 and Ag43-dT digestion products.
- Added 5 ul of NaoAc, 1.5 ul of glycogen and 125 ul of 100% ethanol.
- Centrifuged at 14000 rpm, 30 min at 4C.
- Removed supernatant and added 220 ul of 70% ethanol.
- Centrifuged at 15000 rpm, 15 min at 4C.
- Removed supernatant and dried out at room temperature, after that added 10 ul of DW.
Ligation
Ligation for Ag43-dT as insert and pT7-RBS on pSB1C3 as vector. We use Ligation Mighty Mix (TAKARA BIO INC.) which contains ligase and buffer.
| Vector DNA | 0.25 ul |
| Insert DNA | 3 ul |
| DW | 1.75 ul |
| Ligation Mighty Mix | 5 ul |
| Total | 20 ul |
Ligation reaction time was in detail below.
| Degree | Minute |
| 16 | 30 |
| 65 | 10 |
| 4 | Hold |
Transformation
Transformation for ligation product into DH5α.
- Added 2 ul of DNA to 50 ul of thawed competent cells on ice.
- Incubated on ice for 30 min.
- Added 350 ul of LB.
- Incubated the cells for 2 hours at 37C.
- Prepared and Labeled two plastic plates with LBC.
- Plated 300 ul of the culture onto first dish and spread.
- Added 450 ul of LB to 50 ul of the culture and plated 300 ul of it onto second dish and spread.
- Incubated the plates at 37C for 18 hrs.
 "
"