Team:Paris-Saclay/Project/Notebook/Week 10
From 2012.igem.org
(Difference between revisions)
YohannPetiot (Talk | contribs) |
(→7th August) |
||
| (8 intermediate revisions not shown) | |||
| Line 23: | Line 23: | ||
</head> | </head> | ||
<body> | <body> | ||
| - | <div id="single- | + | <div id="tiles"> |
| - | <div | + | <div id="single-tile3" class="red live-tile" data-mode="flip" data-delay="4000"> |
| - | + | <div> | |
| - | + | <a href="https://2012.igem.org/Team:Paris-Saclay/Project/Abstract"> | |
| - | + | <div class="child-tile"><p class="child-tile">GEMOTE Project</p></div> | |
| + | <img src="https://static.igem.org/mediawiki/2012/7/7c/Project-1.png" alt="" /> | ||
| + | </a> | ||
| + | </div> | ||
<div> | <div> | ||
| - | <img src=" | + | <a href="https://2012.igem.org/Team:Paris-Saclay/Project/Abstract"> |
| - | + | <img src="https://static.igem.org/mediawiki/2012/d/d1/Project2.jpg" alt="" /> | |
| + | <div class="child-tile"><p class="child-tile">GEMOTE Project</p></div> | ||
| + | </a> | ||
</div> | </div> | ||
| - | <script type="text/javascript"> | + | </div> |
| + | <script type="text/javascript"> | ||
// apply regular slide universally unless .exclude class is applied | // apply regular slide universally unless .exclude class is applied | ||
// NOTE: The default options for each liveTile are being pulled from the 'data-' attributes | // NOTE: The default options for each liveTile are being pulled from the 'data-' attributes | ||
$(".live-tile, .flip-list").not(".exclude").liveTile(); | $(".live-tile, .flip-list").not(".exclude").liveTile(); | ||
| - | </script> | + | </script> |
| - | </div> | + | </div> |
</body> | </body> | ||
</html> | </html> | ||
| Line 43: | Line 49: | ||
</div> | </div> | ||
<div id="content-paris-saclay"> | <div id="content-paris-saclay"> | ||
| - | + | ='''Week 10'''= | |
| - | [[Category:Team:Paris-Saclay/Project Gemote| | + | |
| + | [[Category:Team:Paris-Saclay/Project Gemote/Notebook|j]] | ||
__NOTOC__ | __NOTOC__ | ||
====6th August==== | ====6th August==== | ||
| Line 56: | Line 63: | ||
*Liquid culture of B1 in order to prepare a glycerol stock | *Liquid culture of B1 in order to prepare a glycerol stock | ||
| - | ** | + | **Reception of new primers |
**2 Forward | **2 Forward | ||
**3 Reverse | **3 Reverse | ||
**Plasmid Reverse | **Plasmid Reverse | ||
| - | |||
====8th August==== | ====8th August==== | ||
| Line 90: | Line 96: | ||
|- | |- | ||
| style="width: 50%;"|Amplification by PCR of BBa_K274100 already digested by EcoRI. Visualization by electrophoresis on a 0.8% Agarose gel. | | style="width: 50%;"|Amplification by PCR of BBa_K274100 already digested by EcoRI. Visualization by electrophoresis on a 0.8% Agarose gel. | ||
| - | | style="width: 35%;"| [[File:Week10-5.jpg|right| | + | | style="width: 35%;"| [[File:Week10-5.jpg|right|250px]] |
|} | |} | ||
| Line 115: | Line 121: | ||
{{Team:Paris-Saclay/Follow}} | {{Team:Paris-Saclay/Follow}} | ||
| + | </div> | ||
</div> | </div> | ||
</div> | </div> | ||
{{Team:Paris-Saclay/Footer}} | {{Team:Paris-Saclay/Footer}} | ||
Latest revision as of 00:32, 27 September 2012
Week 10
6th August
- Sending of the B1 sample to sequencing with two pairs of primers
- K-274100 Forward and Reverse
- Plasmid pSB1A2 Forward and Reverse
7th August
- Liquid culture of B1 in order to prepare a glycerol stock
- Reception of new primers
- 2 Forward
- 3 Reverse
- Plasmid Reverse
8th August
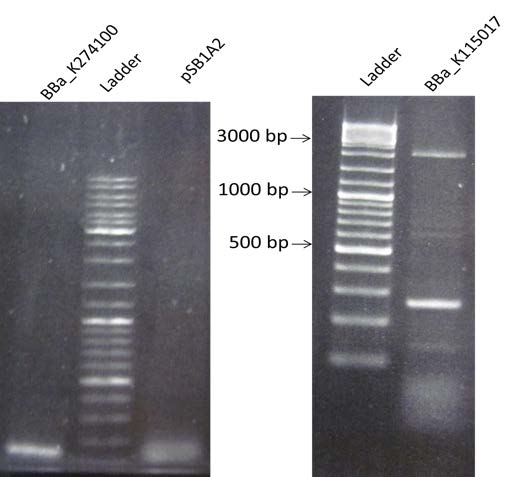
| Amplification of the plasmid pSB1A2, BBa_K274100 and BBa_K115017 by PCR with the new primers. Visualization by electrophoresis on a 2% Agarose gel for BBa_K115017 and a 0.8% Agarose gel for BBa_K274100 and the plasmid pSB1A2. We are expecting a band at 147 bp for BBa_K115017, 3408 bp for BBa_K274100 and 2079 for the plasmid. |
9th August
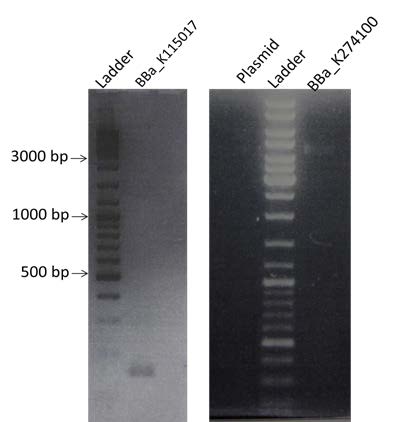
| New amplification by PCR of the plasmid pSB1A2, BBa_K274100 and BBa_K115017 as it has been done the day before. |
- Digestion by EcoRI of the plasmid that contains BBa_K274100.
10th August
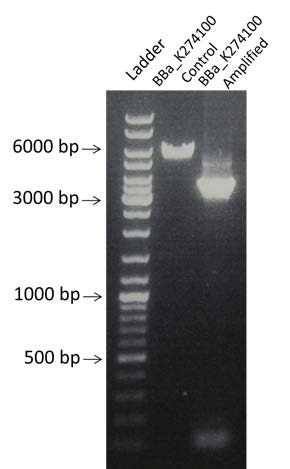
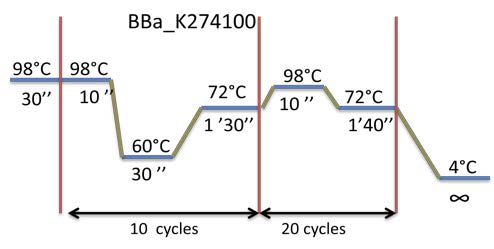
| Amplification by PCR of BBa_K274100 already digested by EcoRI. Visualization by electrophoresis on a 0.8% Agarose gel. |
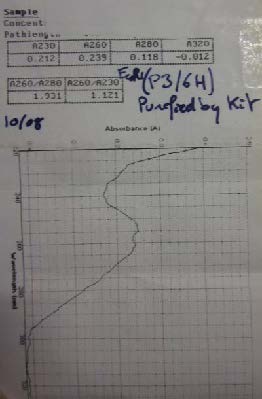
| Miniprep of BBa_K274100 followed by Nanovue to determine the concentration of the sample | |
| Miniprep of the Plasmid pSB1A2 followed by Nanovue to determine the concentration of the sample |
- Digestion by HindIII of the plasmid pSB1A2 in order to linearize it.
| Digestion of BBa_K115017 by DPNI to eliminate the plasmid matrix. Visualization by electrophoresis on a 2% Agarose gel. We are expecting a band at 123 bp. |
 "
"






Follow us !