Team:Frankfurt/New Yeast RFC
From 2012.igem.org
(→Nomenclature and Design of Standardized YBA Primers) |
(→Methods) |
||
| (4 intermediate revisions not shown) | |||
| Line 102: | Line 102: | ||
|h||homologue to | |h||homologue to | ||
|} | |} | ||
| - | Example: | + | Example: fw_PG_aPre10_hpXXX is the name of a forward primer for gene amplification annealing at the BioBrick prefix (BioBrick standard RFC 10) having a homologue overlap to a specific promoter. |
<br><br> | <br><br> | ||
| - | Of course it is possible to design new YBA standard primers for other promoters/terminators of yeast but also for E.coli promoters/terminators. Note that all of these primers must have a specific annealing temperatur in the field of 58 | + | Of course it is possible to design new YBA standard primers for other promoters/terminators of yeast but also for E.coli promoters/terminators. Note that all of these primers must have a specific annealing temperatur in the field of 58 +/- 1°C to combine different primers for every possible PCR amplification. |
All primers for gene amplification must anneal at prefix or suffix of the BioBrick device. The annealing sequence must be compartible to at least BBF RFC 10 standard. Additionally it can be also compartible to other BBF RFC standards. The homologue overlap to the new promoters/terminators must be not less than 40 bp. | All primers for gene amplification must anneal at prefix or suffix of the BioBrick device. The annealing sequence must be compartible to at least BBF RFC 10 standard. Additionally it can be also compartible to other BBF RFC standards. The homologue overlap to the new promoters/terminators must be not less than 40 bp. | ||
| Line 114: | Line 114: | ||
The standardization of primers from YBA standard is only depending on the annealing sequence which must be homologue to the respective BioBrick standard prefix or suffix. The fact that it is insignificant what kind of homologue overlaps the primers have offers the option to choose also other fragments (not only promoters/terminators) incorporating between the BioBrick devices. For assembly of such fragments the suitable homologue overlaps have to be designed onto the primers. | The standardization of primers from YBA standard is only depending on the annealing sequence which must be homologue to the respective BioBrick standard prefix or suffix. The fact that it is insignificant what kind of homologue overlaps the primers have offers the option to choose also other fragments (not only promoters/terminators) incorporating between the BioBrick devices. For assembly of such fragments the suitable homologue overlaps have to be designed onto the primers. | ||
|} | |} | ||
| + | |||
| + | ==Methods== | ||
| + | The main methods you have to use for YBA standard are: <br> | ||
| + | • PCR<br> | ||
| + | • Restriction Digestion for Linearization of a Plasmid <br> | ||
| + | • Control Restriction Digestion of Plasmid with Insert <br> | ||
| + | • Yeast Transformation <br> | ||
| + | • ''E.coli'' Transformation <br> | ||
| + | |||
| + | ==Assembly of ''E.coli'' Vectors== | ||
| + | YBA method can also be used for assembly of ''E.coli'' vectors. Therefore you need a special shuffle plasmid which has an ''E.coli'' promoter and terminator next to the MCS (multiple cloning site) and is also stable in yeast. After that you can design specific primers annealing at the prefix and suffix of your BioBrick device to amplify homologue overlaps to promoters and terminators like it is shown above for yeast expression vectors. Even though ''E.coli'' as a bacteria possesses polycistronic mRNA you have the possibility to regulate every gene by it's own if you assemble additional promoters and terminators between the genes. But you are also able to incorporate other parts, for example other regulatory fragments, between the genes by designing suitable homologue overlaps to these fragments at the primers. | ||
| + | <br><br> | ||
| + | Another possibility is to create primers for one BioBrick device with prefix/suffix annealing sequence and an additional homologue overlap to the coding sequence of a successive BioBrick device. It is important that every second BioBrick device of the insert region must be amplified with specific primers in such a way that only the coding sequence remains. So you can assemble a vector with only one promoter and terminator and a few aligned BioBricks as insert. | ||
| + | <br><br> | ||
| + | After amplifying all fragments in an PCR reaction you transform them into yeast cells. Then you just have to isolate the complete targeting plasmid from yeast, transform it into E.coli and check after another plasmid preparation and a control restriction digestion if it possesses the complete insert. | ||
| + | <br><br> | ||
| + | Up to this point all mentioned primers are designed as standard parts of YBA standard annealing at prefix or suffix of a BioBrick device. More interesting for E.coli vector assembly are the possiblities you have if you are independent of any standard. Then gap repair cloning and homologue recombination system of yeast gets a usefull tool for creating new biobrick devices by combining parts. For example you have the possibility to amplify a RBS (ribosom binding site) to a BioBrick device containing a pure gene. Therefore you have to design a suitable primer paar of respectively app. 20 bp length which must anneale behind of the prefix and in front of the suffix to amplify the pure gene without any BioBrick endings. After that you creat a second primer pair. The forward primer has to anneal at the beginning of the gene having an overlap containing the Shine-Dalgarno-Sequenz/RBS and maybe additionally an homologue overlap to a previous part (e.g. another gene). It is important that you have the pure gene because RBS must be app. 6-7 bp in front of the beginning of the gene. The reverse primer must anneal at the end of the gene and could also have a homologue overlap to a following part. With homologue recombination you can assemble all of your amplified fragments to a completely new BioBrick device by only one yeast transformation. | ||
| + | <br><br> | ||
| + | Moreover at all times you want to assemble a higher number of fragments efficient, fast and with the lowest possible costs homologue recombination in yeast is the better methode compared to restriction digestion and ligation. | ||
| + | ==Full Version of YBA standard== | ||
| + | In the following file the YBA standard is described completely. It also contains sequences of yeast promoters/terminators and suitable YBA standard primers. Additionally all methodes are explained in detail. | ||
| + | |||
| + | [[Media:RFC_final.pdf]] | ||
Latest revision as of 23:33, 26 September 2012

The benefit of vector assembly in yeast
Introduction
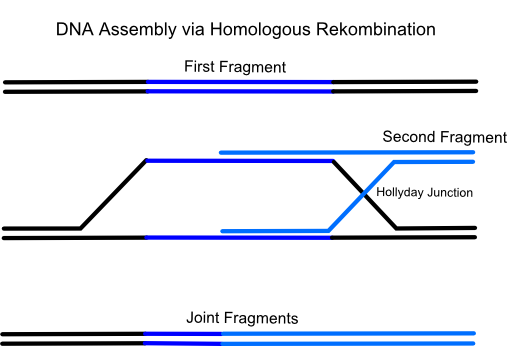
iGEM Team Frankfurt 2012 successfully used a relatively new methode for vector assembly in yeast called Gap Repair Cloning. It is a more and more established methode for efficient, fast and error-free construction of plasmids based on the homologous recombination system of Saccharomyces cerevisiae (common yeast). Naturally yeast uses this process to repair DNA double strand breaks which are one of the most dangerous and life-threatening damages of the DNA for a cell. Therefor this eucaryotic microorganism has developed a few enzymes which have the ability to repair a broken DNA double strand by pairing it with a very similiar DNA region (typically on the homologous chromosome).
Using gap repair cloning a series of linear, successive DNA fragments with homologous overlaps to the respectively following fragment can be transformed in only one step into a yeast cell. After that the micoorganism recombines all fragments in the predetermined, specific order to the final targeting vector. The advantage is that up to eighteen and more successive DNA fragments can be assembled in a single transformation. Also only one restriction enzyme for linearization of the plasmid is needed.
For these reasons iGEM Team Frankfurt thought that gap repair cloning is usefull tool for next iGEM generations. Therefore we developed a standardized methode that describes a new way of assembling BioBrick devices in a desired order to a targeting plasmid using homologue recombination system of yeast. It is called Yeast BioBrick Assembly (YBA). YBA standard only needs one restriction enzyme and a standardized selection of primers and promotors/termintors. It is a continuation of the BioBrick standard and compartible with all BBF RFC 10 parts. Additionally it can be adapted by specific primer design to all other BioBrick standards. In the following we focuse on assembly of yeast expression vectors by using YBA methode. However it also can be used for E.coli vector design or assembly.
Homologue Recombination System of Yeast
There are many endogenous and exogenous factors (for example reactive oxygen-species, ionizing radiation, chemicals and failing of DNA binding enzymes (e.g. collapsed replication forks)) which causes DNA double strand breaks. For the cell this is the most dangerous DNA damage because even if it occurs in rather unimportant regions the cell will not survive the next cell cycle. That's the reason why yeast possesses highly active enzymes which have the ability to repair a broken double strand by pairing it with a very similiar DNA region (typically on the homologous chromosome). This process is called homologous recombination. Using the gap repair method this natural process can be exploited for the construction of large cloning vectors in yeast.
Design of DNA fragments for gap repair cloning
The idea of the method is to transform a series of linear, successive DNA fragments into one yeast cell. The linear fragments have open blunt ends like they occur after a double strand break. If a homologous sequence is available it will be treated like a genomic double strand break and homologous recombination takes place. When the successive DNA fragments are designed in a specific way which includes large sequence overlaps (overall app. 40 bp) to the respectively following fragment yeast will recombinate them together.For the formation of a cloning vector the first fragment is a yeast-E.coli shuttle plasmid which is linearized by an appropriate restriction digest. A shuttle plasmid is a plasmid which is stable both in yeast and in Escherichia coli. The first fragment of the insert has to possess an homologous overlap to both the wished insertion site on the plasmid and to the beginning of the second fragment. The end of the second fragment has to possess an overlap to the beginning of the third one and so on. At least the end of the last fragment of the insert again has to possess an overlap homologous to the second insertion site on the plasmid.
At the lab of our instructor up to eighteen single fragments were assembled in a single transformation. Another advantage of the method is that no scars are left between the inserted fragments. Assembly of fragments to joint genes is possible. Restriction enzymes only have to be used once for linearization of the shuttle plasmid.
Example from our project
Yeast BioBrick Assembly - Gap repair cloning for iGEM
Assembly of Yeast Expression Vector Using YBA Standard
Nomenclature and Design of Standardized YBA Primers
| Abbreviation | Meaning |
|---|---|
| p | Primer |
| PG | Primer for Gene Amplification |
| PTP | Primer for Terminator-Promoter Part Amplification |
| PP | Primer for Promoter Amplification |
| PT | Primer for Terminator Amplification |
| fw | forward Primer |
| rev | reverse Primer |
| p | Promoter |
| t | Terminator |
| Pre10 | Prefix BioBrick RFC 10 |
| Suf10 | Suffix BioBrick RFC 10 |
| a | annealing at |
| h | homologue to |
Example: fw_PG_aPre10_hpXXX is the name of a forward primer for gene amplification annealing at the BioBrick prefix (BioBrick standard RFC 10) having a homologue overlap to a specific promoter.
Of course it is possible to design new YBA standard primers for other promoters/terminators of yeast but also for E.coli promoters/terminators. Note that all of these primers must have a specific annealing temperatur in the field of 58 +/- 1°C to combine different primers for every possible PCR amplification.
All primers for gene amplification must anneal at prefix or suffix of the BioBrick device. The annealing sequence must be compartible to at least BBF RFC 10 standard. Additionally it can be also compartible to other BBF RFC standards. The homologue overlap to the new promoters/terminators must be not less than 40 bp.
Primers for amplification of singel promoters or terminators as well as for ligated terminator-promoter parts must anneal behind the prefix or in front of the suffix, except of the forward primer of single promoter parts which is intended to be the only one having an homologue overlap of 40 bp to previous terminators. The homologue overlap of this primer is essential for possibility of combining own terminator-promoter parts.
Pay attention that you must design two primers (forward and reverse) for every PCR reaction. Each primer must have a free 3'-ending in direction of the DNA fragment so the DNA-Polymerase can synthesise the new strand in 5' to 3' direction. The consequence of this is that the forward primer must anneal to the 3'-5' singel strand DNA and the reverse primer must anneal to the 5'-3' single strand DNA of the template.
The standardization of primers from YBA standard is only depending on the annealing sequence which must be homologue to the respective BioBrick standard prefix or suffix. The fact that it is insignificant what kind of homologue overlaps the primers have offers the option to choose also other fragments (not only promoters/terminators) incorporating between the BioBrick devices. For assembly of such fragments the suitable homologue overlaps have to be designed onto the primers.
Methods
The main methods you have to use for YBA standard are:
• PCR
• Restriction Digestion for Linearization of a Plasmid
• Control Restriction Digestion of Plasmid with Insert
• Yeast Transformation
• E.coli Transformation
Assembly of E.coli Vectors
YBA method can also be used for assembly of E.coli vectors. Therefore you need a special shuffle plasmid which has an E.coli promoter and terminator next to the MCS (multiple cloning site) and is also stable in yeast. After that you can design specific primers annealing at the prefix and suffix of your BioBrick device to amplify homologue overlaps to promoters and terminators like it is shown above for yeast expression vectors. Even though E.coli as a bacteria possesses polycistronic mRNA you have the possibility to regulate every gene by it's own if you assemble additional promoters and terminators between the genes. But you are also able to incorporate other parts, for example other regulatory fragments, between the genes by designing suitable homologue overlaps to these fragments at the primers.
Another possibility is to create primers for one BioBrick device with prefix/suffix annealing sequence and an additional homologue overlap to the coding sequence of a successive BioBrick device. It is important that every second BioBrick device of the insert region must be amplified with specific primers in such a way that only the coding sequence remains. So you can assemble a vector with only one promoter and terminator and a few aligned BioBricks as insert.
After amplifying all fragments in an PCR reaction you transform them into yeast cells. Then you just have to isolate the complete targeting plasmid from yeast, transform it into E.coli and check after another plasmid preparation and a control restriction digestion if it possesses the complete insert.
Up to this point all mentioned primers are designed as standard parts of YBA standard annealing at prefix or suffix of a BioBrick device. More interesting for E.coli vector assembly are the possiblities you have if you are independent of any standard. Then gap repair cloning and homologue recombination system of yeast gets a usefull tool for creating new biobrick devices by combining parts. For example you have the possibility to amplify a RBS (ribosom binding site) to a BioBrick device containing a pure gene. Therefore you have to design a suitable primer paar of respectively app. 20 bp length which must anneale behind of the prefix and in front of the suffix to amplify the pure gene without any BioBrick endings. After that you creat a second primer pair. The forward primer has to anneal at the beginning of the gene having an overlap containing the Shine-Dalgarno-Sequenz/RBS and maybe additionally an homologue overlap to a previous part (e.g. another gene). It is important that you have the pure gene because RBS must be app. 6-7 bp in front of the beginning of the gene. The reverse primer must anneal at the end of the gene and could also have a homologue overlap to a following part. With homologue recombination you can assemble all of your amplified fragments to a completely new BioBrick device by only one yeast transformation.
Moreover at all times you want to assemble a higher number of fragments efficient, fast and with the lowest possible costs homologue recombination in yeast is the better methode compared to restriction digestion and ligation.
Full Version of YBA standard
In the following file the YBA standard is described completely. It also contains sequences of yeast promoters/terminators and suitable YBA standard primers. Additionally all methodes are explained in detail.
 "
"