Team:NRP-UEA-Norwich/Parts
From 2012.igem.org
Khadijaouadi (Talk | contribs) (→The Comparator Circuit) |
Khadijaouadi (Talk | contribs) (→Parts to characterise in the future) |
||
| (29 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
{{UEANRP}} | {{UEANRP}} | ||
| - | |||
| - | |||
| - | == | + | ==Our Parts== |
| - | + | We truly believe in the iGEM ethos of maintaining a Registry of Parts of constantly improving BioBricks and feel that, while creating new entirely new BioBricks is required for the registry to grow, the further characterisation and improvement of BioBricks that already exist is the key to maintaining the registry's credibility. | |
| - | |||
| - | + | <groupparts>iGEM012 NRP-UEA-Norwich</groupparts> | |
| - | + | <br><br> | |
| - | + | == Characterisation of other parts == | |
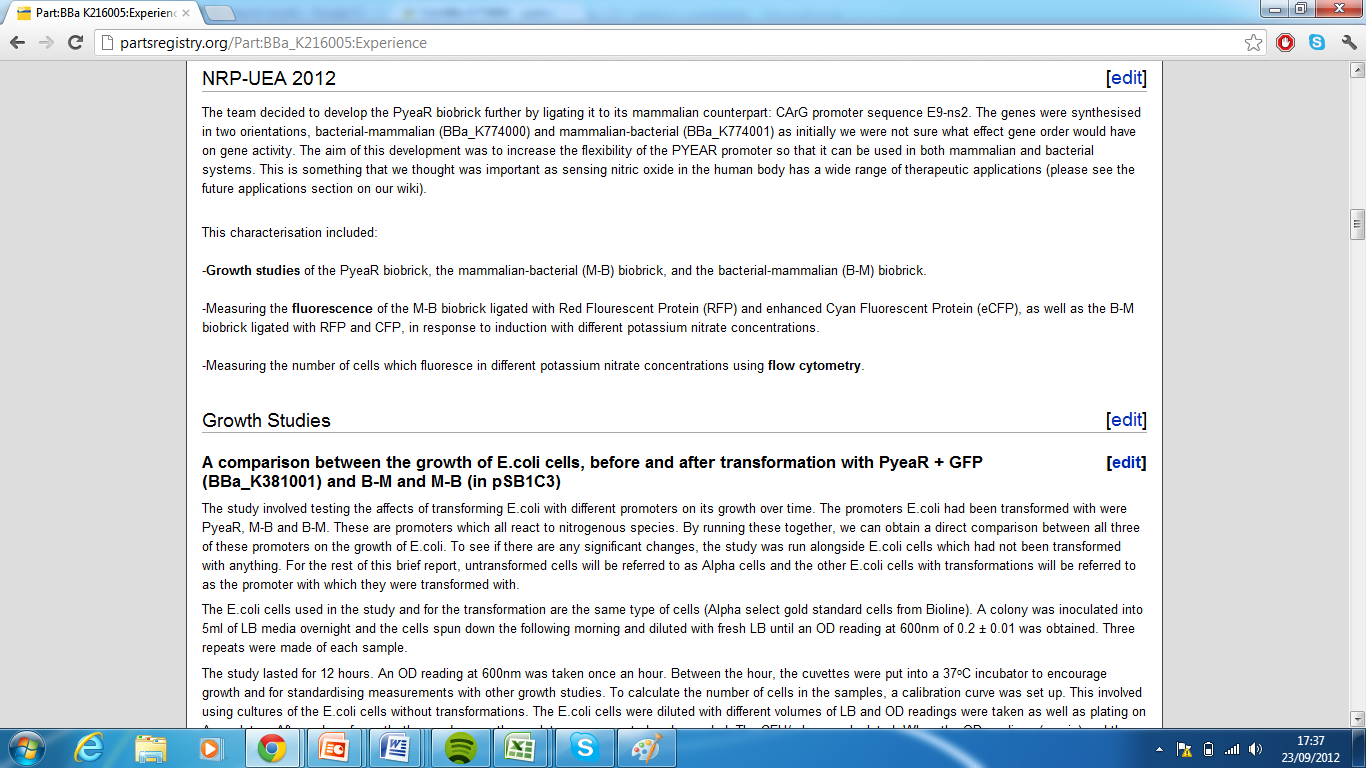
| - | + | 1) Part:BBa_K216005:Please see experience page | |
| + | [http://partsregistry.org/Part:BBa_K216005:Experience] (Group: iGEM09_Edinburgh (2009-09-25)) | ||
| + | [[File:PYEAR_page.png|800px]] | ||
| - | + | <br><br> | |
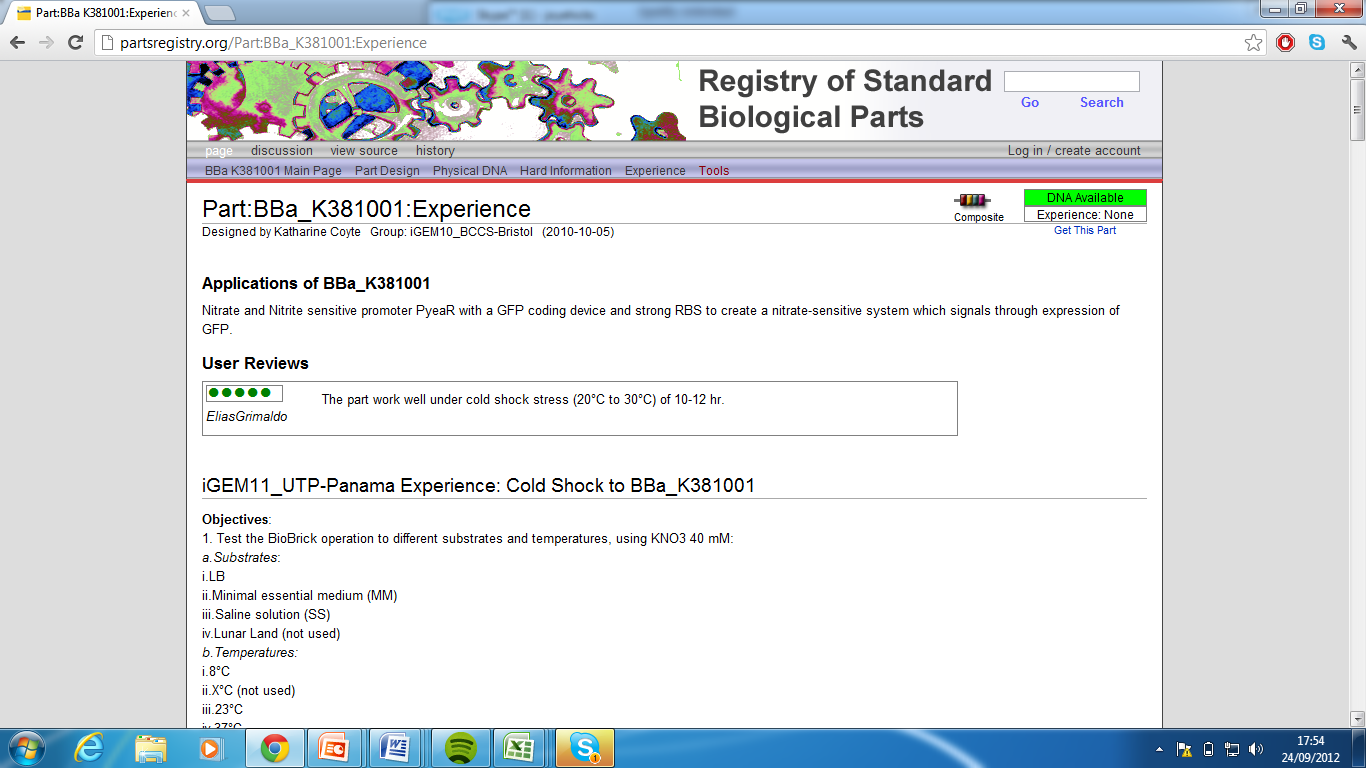
| + | 2) Part:BBa_K381001:Please see experience page [http://partsregistry.org/Part:BBa_K381001:Experience] (Group: iGEM10_BCCS-Bristol (2010-10-05)) | ||
| + | [[File:K381001.png|800px]] | ||
| + | <br><br> | ||
| - | == | + | == Characterisation of our parts == |
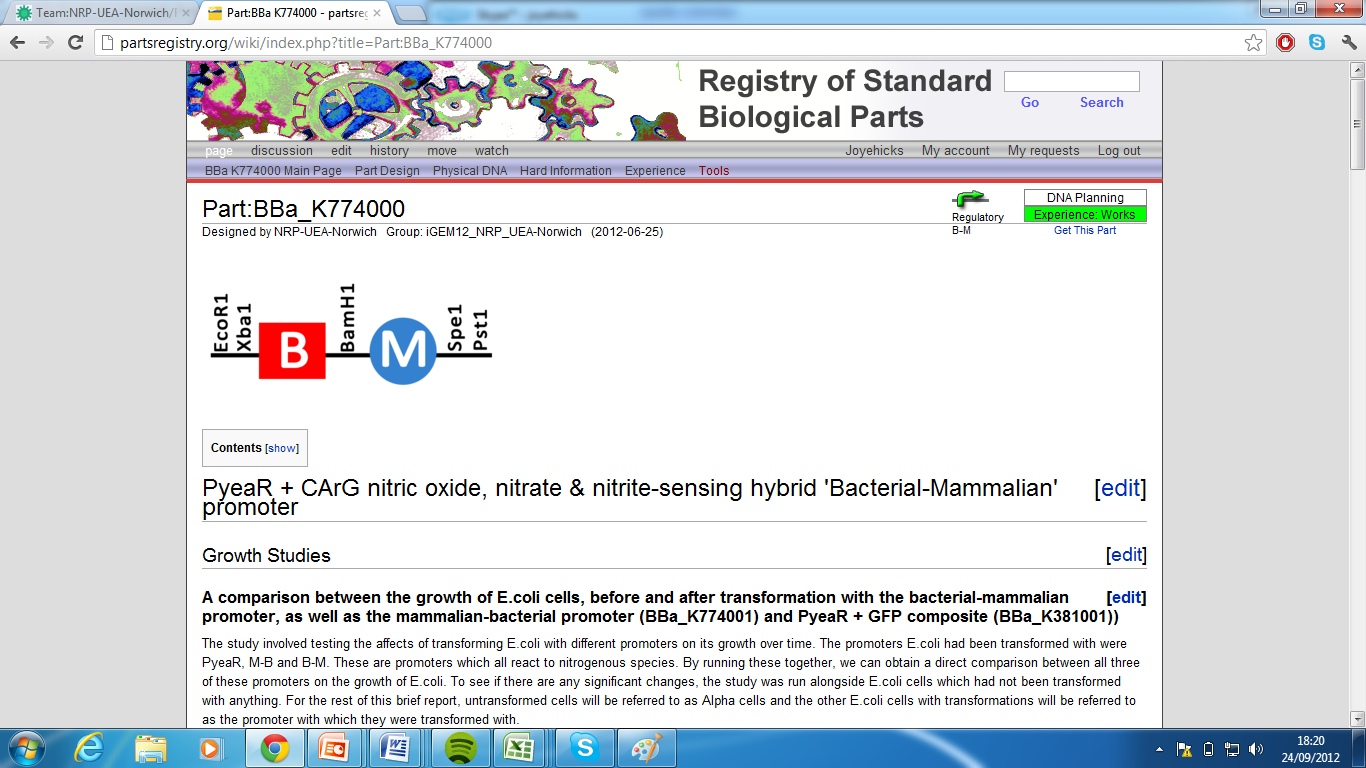
| + | 1)Bacterial-Mammalian Promoter, Part:BBa_K774000[http://partsregistry.org/wiki/index.php?title=Part:BBa_K774000] | ||
| + | [[File:4000.png|800px]] | ||
| + | <br><br> | ||
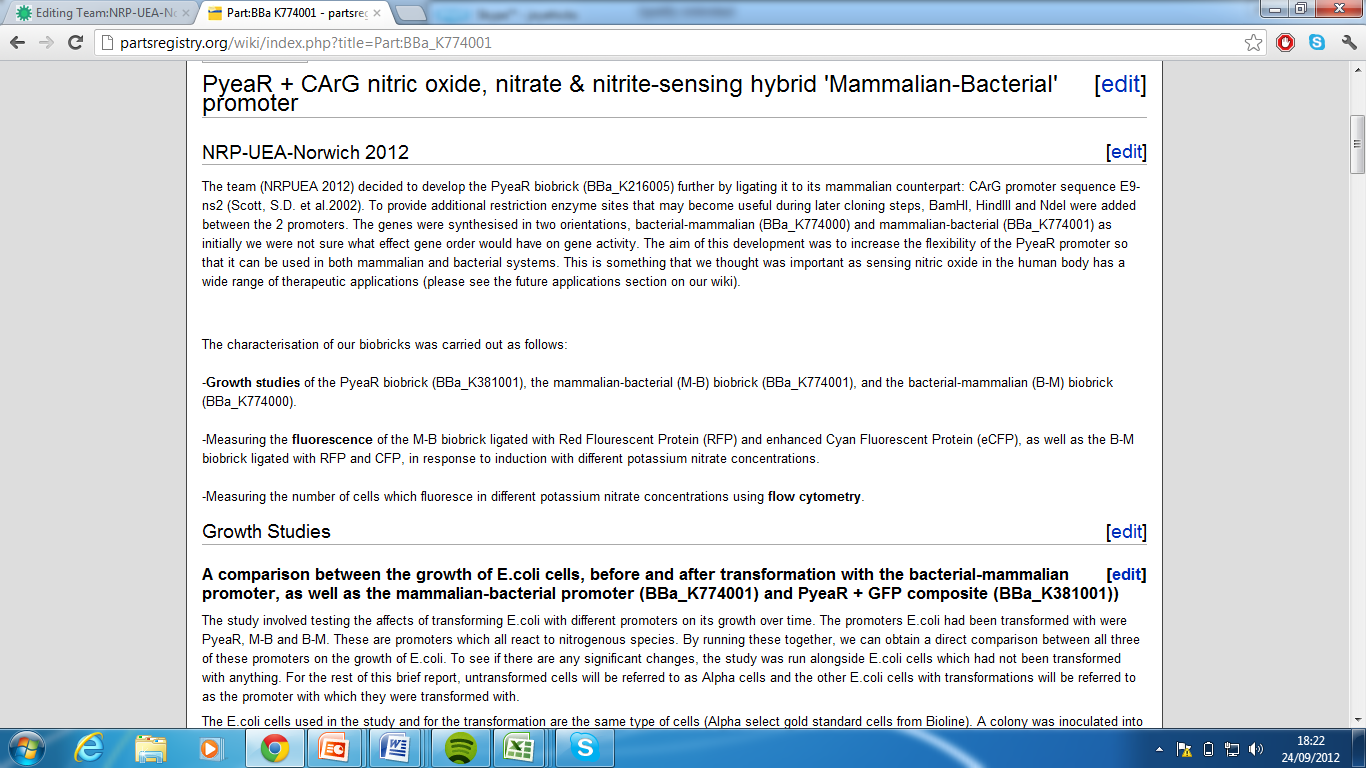
| + | 2)Mammalian-Bacterial Promoter, Part:BBa_K774001[http://partsregistry.org/wiki/index.php?title=Part:BBa_K774001] | ||
| + | [[File:001.png|800px]] | ||
| + | <br><br> | ||
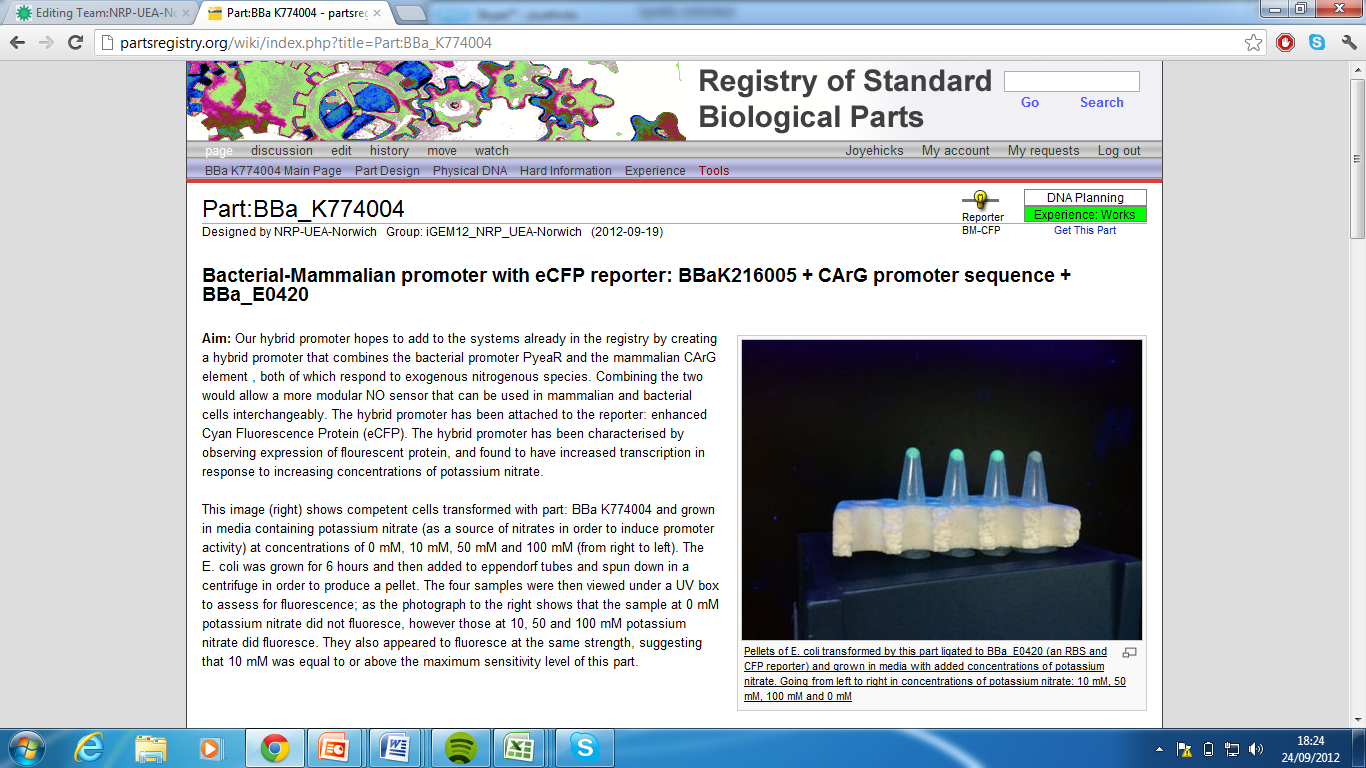
| + | 3)Bacterial-Mammalian promoter with eCFP reporter, Part:BBa_K774004[http://partsregistry.org/wiki/index.php?title=Part:BBa_K774004] | ||
| + | [[File:004.png|800px]] | ||
| + | <br><br> | ||
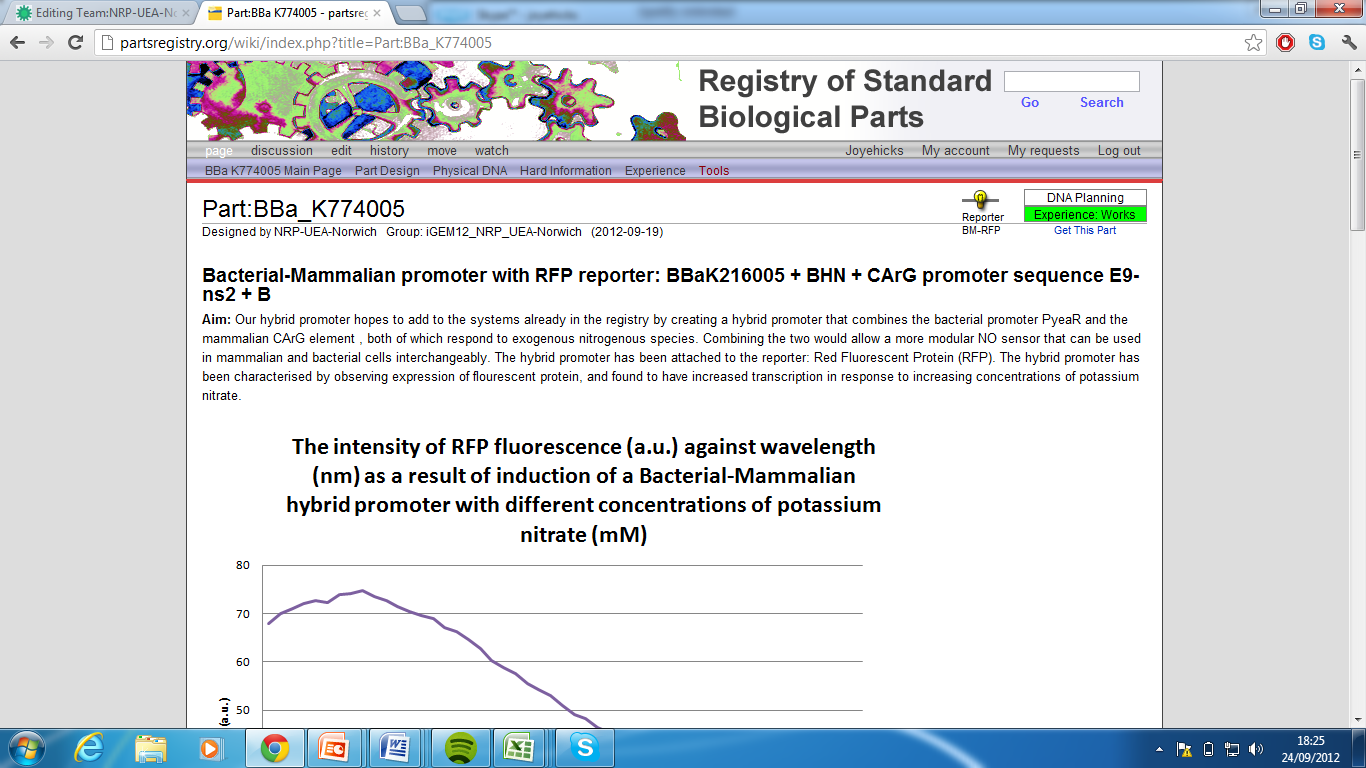
| + | 4)Bacterial-Mammalian promoter with RFP reporter,Part:BBa_K774005[http://partsregistry.org/wiki/index.php?title=Part:BBa_K774005] | ||
| + | [[File:005.png|800px]] | ||
| + | <br><br> | ||
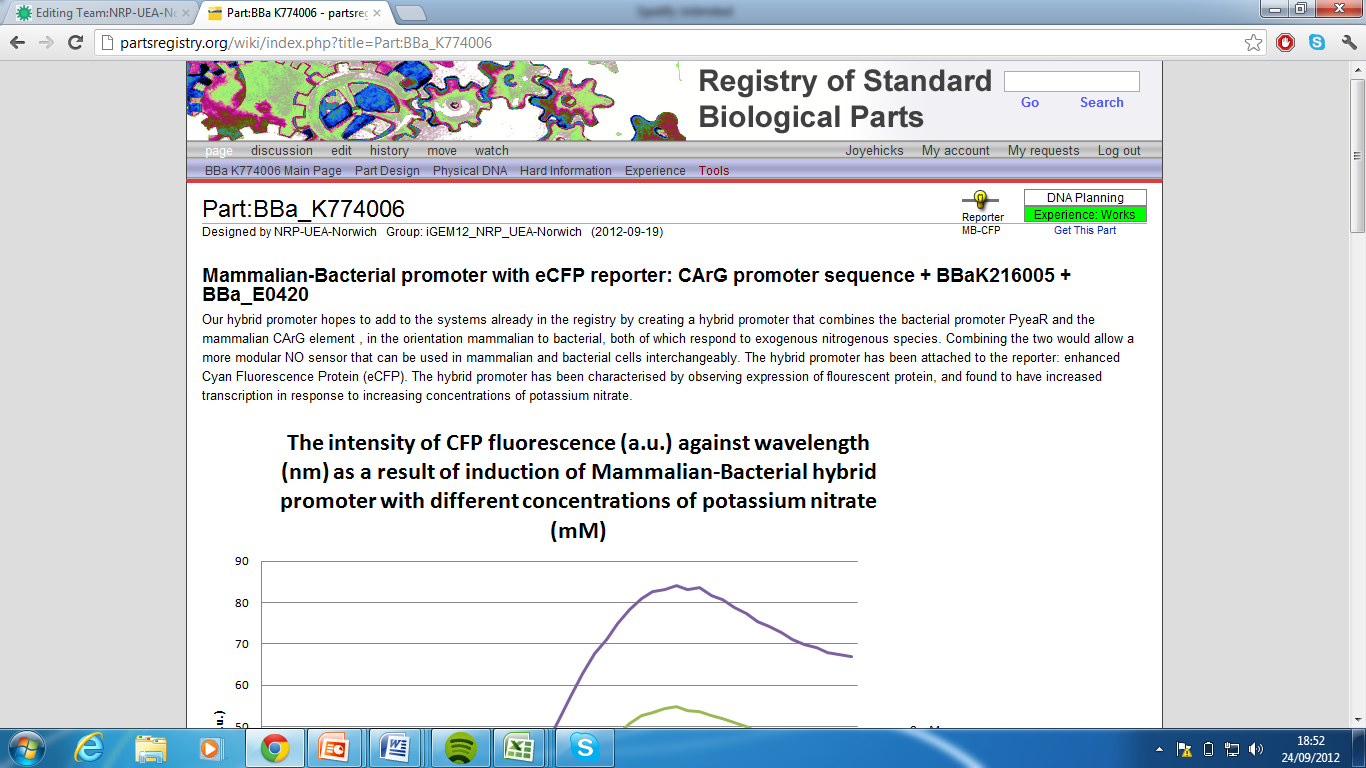
| + | 5)Mammalian-Bacterial promoter with eCFP reporter, Part:BBa_K774006[http://partsregistry.org/wiki/index.php?title=Part:BBa_K774006] | ||
| + | [[File:006.png|800px]] | ||
| + | <br><br> | ||
| + | 6)Mammalian-Bacterial promoter with RFP reporter, Part:BBa_K774007[http://partsregistry.org/wiki/index.php?title=Part:BBa_K774007] | ||
| + | [[File:007.png|800px]] | ||
| - | < | + | == Parts to characterise in the future == |
| + | 1)Comparator Circuit Part 1, Part:BBa_K774002 | ||
| + | <br><br> | ||
| + | 2)Comparator Circuit Part 2, Part:BBa_K774003 | ||
| + | <br><br> | ||
| + | Lack of specificity is a problem which many of the parts in the parts registry suffer from, and certainly a challenge which we faced when trying to detect nitrogenous species. From this potential problem spawned a potential solution; the Comparator Circuit. The two BioBricks form a pair which are designed to specifically bind to each other while ligated to two different promoters of overlapping specificity. This results in an integrating of the conflicting outputs of the two opposing gene systems. | ||
| + | |||
| + | Our system relies on two constructs that interact via complimentary base pair sequences both before and after the ribosome binding site of the reporter protein. The idea being that, when both transcripts are present in the chassis, they would bind together, inhibiting the translation of the reporter proteins. | ||
| + | Any imbalance of transcription due to the presence of the substrate of interest results in free mRNA of the gene system that detects that substrate. Crucially, if both promoters detect the same substrates but differ with one extra substrate being detected by one of the promoters, it is this substrate and this substrate only that our system will be able to detect in a simple and quantitative way. | ||
| + | |||
| + | Our team have constructed a countercurrent comparator circuit in which the reporter proteins are at the same end of the complimentary region, although a contracurrent system has been theorised. Both systems share a crucial subtractive nature comparable to an analogue computer. We envisage that, should the system be fine-tuned and expanded on, a variety of different business sectors from agriculture to spinoff pharmaceutical companies (such as the fictious QuantaCare which you can read about on our wiki) could capitalise on this novel genetic technology. | ||
| + | What we have produced is a BioBrick pair that work in harmony, when ligated to promoters of interest and genes of interest, to sequester translation when both mRNA transcripts are present in the cell. The use of quantitative tuners with these BioBricks is encouraged to ensure that the transcription rate both gene constructs are equal when both promoters are transcribing at their optimal rate. Although the parts have been submitted to the registry and theoretically characterised, time constraints have meant that further lab-based characterisation could not occur. | ||
| + | However, we hope to utilise any free time in our timetables during the next semester to characterise the BioBricks further (please see our project proposal), and hope that we will be given a chance to present our further findings at MIT! | ||
| + | |||
| + | To conclude, what we have created is a pair of antagonistic BioBricks that turned the pair of mRNAs in which they reside into translational repressor molecules when both are transcribed in tandum within a specific chassis of interest, a new application for mRNA complimentary base pairing within the registry and a project we feel could go very far indeed. | ||
Latest revision as of 23:20, 26 September 2012
 "
"