Team:SJTU-BioX-Shanghai/Project/project1.1
From 2012.igem.org
(Difference between revisions)
AleAlejandro (Talk | contribs) (→Membrane Protein Construction) |
AleAlejandro (Talk | contribs) (→Membrane Localization) |
||
| Line 34: | Line 34: | ||
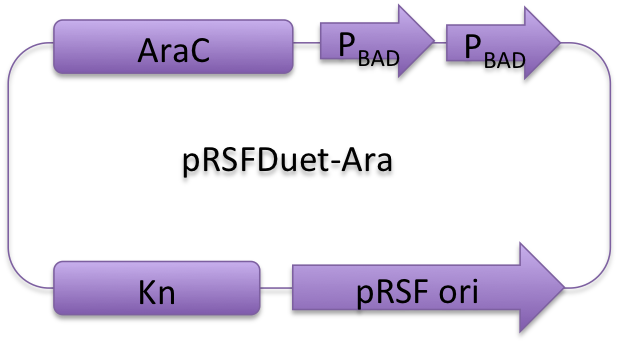
All proteins we used are inserted into those three reconstructed vectors and under control of araBAD promoter. | All proteins we used are inserted into those three reconstructed vectors and under control of araBAD promoter. | ||
[[Image:12SJTU-pACYC.png|240px|left]][[Image:12SJTU-pET.png|240px]][[Image:12SJTU-pRSF.png|240px]] | [[Image:12SJTU-pACYC.png|240px|left]][[Image:12SJTU-pET.png|240px]][[Image:12SJTU-pRSF.png|240px]] | ||
| - | '' | + | ''Fig. 1:'' Profile of reconstructed plasmids in ''Membrane Magic'' Project |
<br> | <br> | ||
<br> | <br> | ||
| Line 42: | Line 42: | ||
===Membrane Protein Construction=== | ===Membrane Protein Construction=== | ||
| - | [[Image:12SJTU_proteinconstruction.jpg|thumb|600px|center|'' | + | [[Image:12SJTU_proteinconstruction.jpg|thumb|600px|center|''Fig.2:'' Construction sketch of membrane assemblies]] |
To construct membrane assemblies, we must make sure that our device is directed to inner membrane of ''E.coli''. | To construct membrane assemblies, we must make sure that our device is directed to inner membrane of ''E.coli''. | ||
| Line 63: | Line 63: | ||
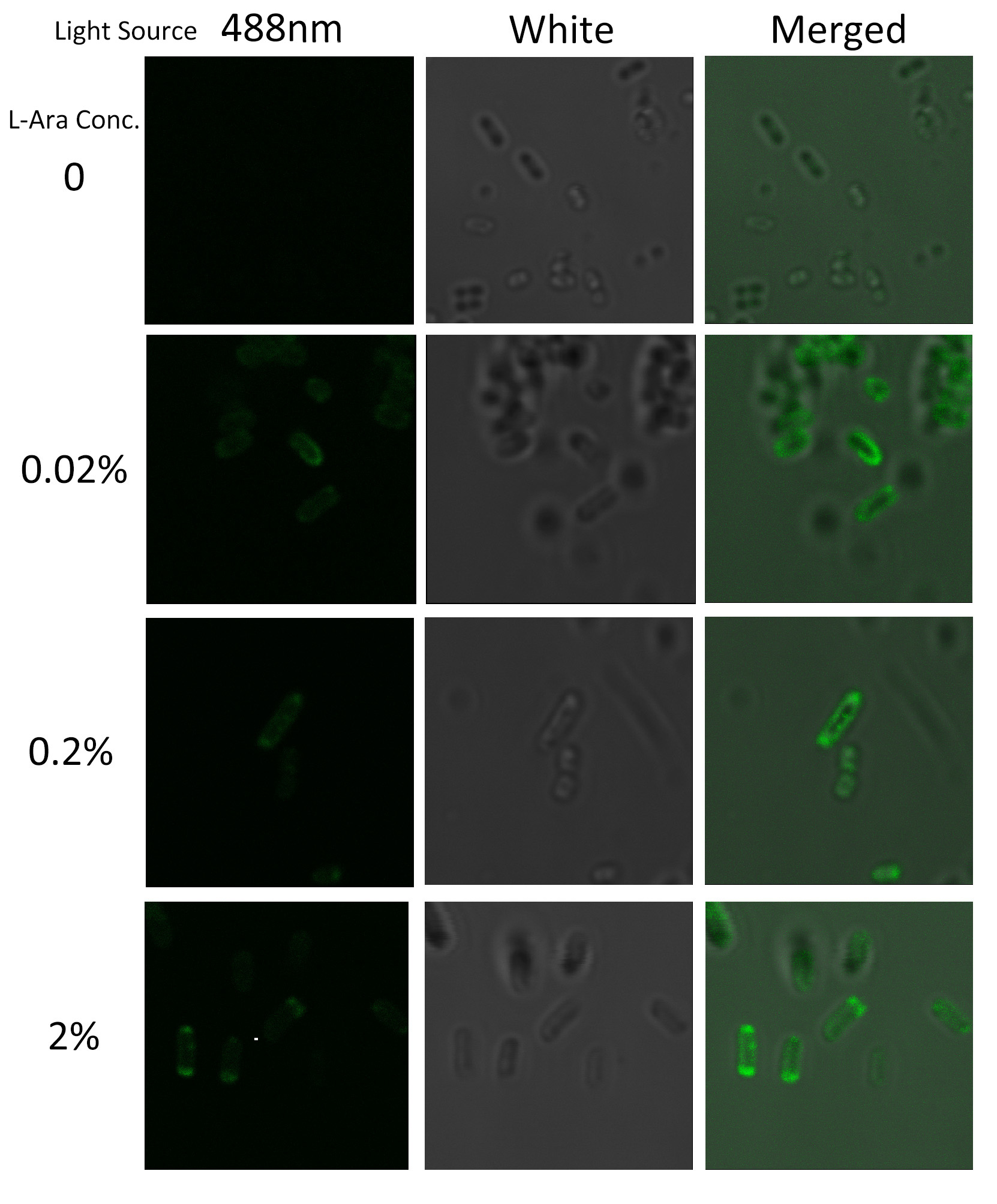
To visualize the localization of BlaLG fusion protein, we adopted fluorescence test. GFP fused to the C terminus of BlaLG enables us to have a closer look at where the membrane proteins are localized. Under laser confocal microscope, we can observe the location of the fluorescence, thus to confirm the exact subcellular localization of the fusion protein. | To visualize the localization of BlaLG fusion protein, we adopted fluorescence test. GFP fused to the C terminus of BlaLG enables us to have a closer look at where the membrane proteins are localized. Under laser confocal microscope, we can observe the location of the fluorescence, thus to confirm the exact subcellular localization of the fusion protein. | ||
| - | [[Image:12SJTU_MLGFP.jpg|thumb|500px|center|''Fig. | + | [[Image:12SJTU_MLGFP.jpg|thumb|500px|center|''Fig.4'' :Bacteria carrying BlaLG induced at different concentration of L-arabinose.]] |
It is observed that green fluorescence intensity of ''E.coli'' margin is higher than that of cytoplasm. ''Fig.3'' proves again that BlaLG has been localized to membrane. | It is observed that green fluorescence intensity of ''E.coli'' margin is higher than that of cytoplasm. ''Fig.3'' proves again that BlaLG has been localized to membrane. | ||
| Line 78: | Line 78: | ||
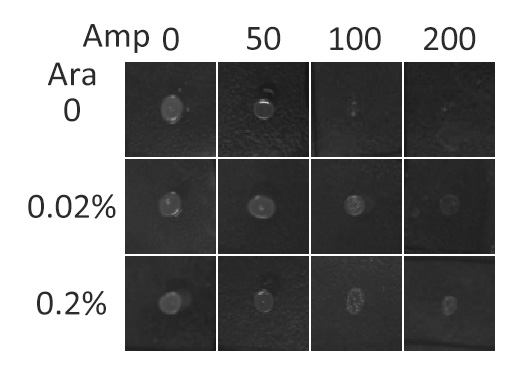
β-lactamase,a bacterial enzyme which must be exported to the periplasm in order to confer significant resistance to β-lactam antibiotics, such as ampicillin. Note that N-terminus of Lgt faces the periplasm and C-terminus faces the cytoplasm。 Hence, if our fusion membrane protein is correctly anchored to membrane,β-lactamase is expected to be functional and host cells should be able to grow on culture media containing ampicillin. (Skretas and Georgiou 2010) | β-lactamase,a bacterial enzyme which must be exported to the periplasm in order to confer significant resistance to β-lactam antibiotics, such as ampicillin. Note that N-terminus of Lgt faces the periplasm and C-terminus faces the cytoplasm。 Hence, if our fusion membrane protein is correctly anchored to membrane,β-lactamase is expected to be functional and host cells should be able to grow on culture media containing ampicillin. (Skretas and Georgiou 2010) | ||
| - | [[Image:12SJTU_AntiTest.jpg|thumb|400px|center|''Fig. | + | [[Image:12SJTU_AntiTest.jpg|thumb|400px|center|''Fig.5'' :Growth phenotypes of ''E. coli'' cells expressing BlaLG on LB agar media with concentration of inducer L-arabinose from 0 to 0.2%. We increased the concentration of ampicillin from 0 to 200 (μg/ ml).Bacteria carrying BlaLG were able to grow at ampicillin concentration of 200 μg/ ml with induction]] |
| - | ''Fig. | + | ''Fig.5'' showed that our fusion protein has been correctly localized to membrane. Besides, a very low L-arabinose concentration at 0.02% is already enough to induce sufficient amount of membrane protein. |
| Line 86: | Line 86: | ||
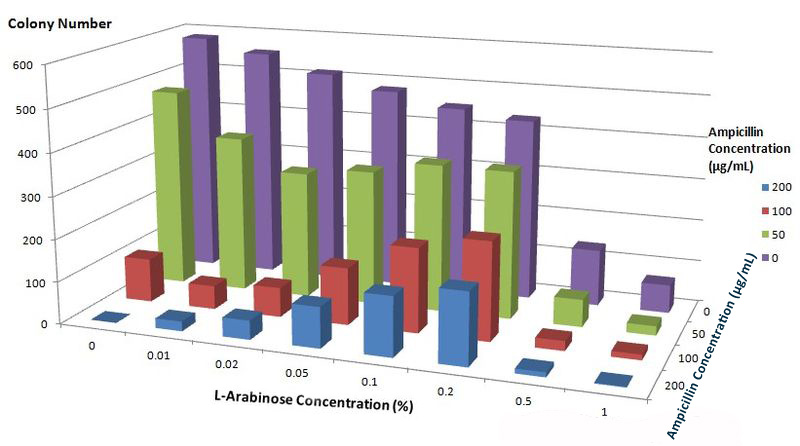
| - | [[Image:12SJTU_Antibioticstest2.jpg|thumb|600px|center|''Fig. | + | [[Image:12SJTU_Antibioticstest2.jpg|thumb|600px|center|''Fig.6'' :Growth condition of ''E. coli'' cells expressing BlaLG on LB agar media with concentration of L-arabinose and Ampicillin. Single colony is picked and cultivated at 37℃ until OD value reaches 0.7. Bacteria cultures are diluted by 1:100000 and coated onto plates containing different concentration of L-Arabinose and Ampicillin. Growth condition is measured through counting colonies on plates.]] |
The result apparently showed that at L-Arabinose concentration of 0.1% and 0.2%, more bacteria could grow on high concentration of Ampicllin. Thus, L-Arabinose concentration of 0.1% and 0.2 best suits membrane protein expression in Project ''Membrane Magic''. | The result apparently showed that at L-Arabinose concentration of 0.1% and 0.2%, more bacteria could grow on high concentration of Ampicllin. Thus, L-Arabinose concentration of 0.1% and 0.2 best suits membrane protein expression in Project ''Membrane Magic''. | ||
Revision as of 14:23, 26 September 2012
 "
"