Team:LMU-Munich/Data/Inducible
From 2012.igem.org
| Line 5: | Line 5: | ||
| - | [[File:plate reader induzierbar promotor.png|thumb|right|400px|<p align="justify">'''Fig. 1: Luminescence measurements of the inducible ''B. subtilis'' promoter P<sub>''liaI''</sub> after induction with different concentrations of bacitracin.''' OD600 (up), luminescence (middle), and luminescence/OD600 (down) of the two clones (green/blue) depending on the time (h) after induction with bacitracin (0 μg/ml(left) to 100μg/ml(right). Data derive from three independent experiments, the graphs show the mean fo the three experiments and the standard deviation. Curves were fitted over each other (t=0, OD<sub>''600''</sub>=0,3) and smoothed by taking the average of three neighboring values. t=0 is the time of induction. | + | [[File:plate reader induzierbar promotor.png|thumb|right|400px|<p align="justify">'''Fig. 1: Luminescence measurements of the inducible ''B. subtilis'' promoter P<sub>''liaI''</sub> after induction with different concentrations of bacitracin.''' OD600 (up), luminescence (middle), and luminescence/OD600 (down) of the two clones (green/blue) depending on the time (h) after induction with bacitracin (0 μg/ml(left) to 100μg/ml(right)). Data derive from three independent experiments, the graphs show the mean fo the three experiments and the standard deviation. Curves were fitted over each other (t=0, OD<sub>''600''</sub>=0,3) and smoothed by taking the average of three neighboring values. t=0 is the time of induction.</p> ]] |
<p align="justify"> | <p align="justify"> | ||
The inducible promoter '''P<sub>''liaI''</sub>''' was evaluated in the reporter vector pSB<sub>Bs</sub>3C-<i>luxABCDE</i> which contains the ''lux'' operon [[File:Lux operon.png|100px]]. This is why the promoter activity leads to gene expression and to the production of the protein luciferase. The luminescence of this protein can be measured with the plate reader ''Synergy2'' (Biotek) ('''Fig.1'''). All clones show a normal growth behaviour up to a bacitracin concentration of 10 μg/ml. At higher concentrations of bacitracin the growth curves decrease because the cells die in presence of bacitracin. The promoter PliaI shows a basal activity level of about 10.000 Lumi/OD600 at the maximum. After induction with bacitracin the luminescence per OD600 increases depending of the concentration of bacitracin. The highest activity of about 1.5 Mio Lumi/OD600 can be measured after induction with 10-30 μg/ml bacitracin. If the concentration gets higher than 100 μg/ml the luminescence of both clones show a different behaviour. To see also the induction of this inducible promoter in comparison to an uninducible promoter we also did induction experiments with PliaG which should not be inducible. As expected there is no response in activity depending on the induction and PliaG shows a constant maximum of activity of about 10.000 Lumi/OD600 (Data not shown).</p> | The inducible promoter '''P<sub>''liaI''</sub>''' was evaluated in the reporter vector pSB<sub>Bs</sub>3C-<i>luxABCDE</i> which contains the ''lux'' operon [[File:Lux operon.png|100px]]. This is why the promoter activity leads to gene expression and to the production of the protein luciferase. The luminescence of this protein can be measured with the plate reader ''Synergy2'' (Biotek) ('''Fig.1'''). All clones show a normal growth behaviour up to a bacitracin concentration of 10 μg/ml. At higher concentrations of bacitracin the growth curves decrease because the cells die in presence of bacitracin. The promoter PliaI shows a basal activity level of about 10.000 Lumi/OD600 at the maximum. After induction with bacitracin the luminescence per OD600 increases depending of the concentration of bacitracin. The highest activity of about 1.5 Mio Lumi/OD600 can be measured after induction with 10-30 μg/ml bacitracin. If the concentration gets higher than 100 μg/ml the luminescence of both clones show a different behaviour. To see also the induction of this inducible promoter in comparison to an uninducible promoter we also did induction experiments with PliaG which should not be inducible. As expected there is no response in activity depending on the induction and PliaG shows a constant maximum of activity of about 10.000 Lumi/OD600 (Data not shown).</p> | ||
| - | + | <br> | |
| - | [[File:Englisch Auswertung PliaI.png|thumb|right|400px|<p align="justify">'''Fig. 2: β-galactosidase assay | + | <br> |
| + | <br> | ||
| + | <br> | ||
| + | [[File:Englisch Auswertung PliaI.png|thumb|right|400px|<p align="justify">'''Fig. 2: β-galactosidase assay with (grey) and without (black) induction of bacitracin (20μg/ml) of strains carrying the promoter P<sub>''liaI''</sub> fused to ''lacZ'''''. The average of the β-galactosidase activity (Miller Units) and the standard deviation of two independent clones are shown depending on the time(minutes after induction). Experiment shows representative data which was obtained in the same way from three independent experiments.</p>]] | ||
<p align="justify"> | <p align="justify"> | ||
| Line 20: | Line 23: | ||
</p> | </p> | ||
| + | Der β-Galaktosidase Assay des induzierbaren B. subtilis-Promotors PliaI wurde wie in 2.5.4. beschrieben mit dem Stamm iGEMB13 mit und ohne Induktion durch Bacitracin (20μg/ml) bei einer OD600 von 0,4 durchgeführt. Dabei wird durch den entstandenen gelben Farbstoff auf die Enzymmenge an β-Galaktosidase und somit auf die Promotoraktivität, die für die Stärke der Expression verantwortlich ist, geschlossen. Die Ergebnisse einer Messung, die sich in drei unabhängigen Experimenten bestätigten, sind in Abb. 9 gezeigt. Der Promotor PliaI zeigt ohne Induktion durch Bacitracin keine Aktivität. Nach Induktion durch Bacitracin (20μg/ml) ist die Expression der β-Galaktosidase sehr stark, wodurch so viel Enzym entsteht, dass die gesamte Aktivität des Enzyms bereits nach 30 Minuten etwa 200 Miller Units erreicht, bevor sie auf ein Maximum von etwa 500 Miller Units ansteigt (t=60 Min). Somit ist die Aktivität des Enzyms und somit des Promotors nach Induktion mit dieser Bacitracin-Konzentration an ihrem Maximum etwa 500 Mal größer als ohne Induktion. | ||
Revision as of 09:20, 24 September 2012
The LMU-Munich team is exuberantly happy about the great success at the World Championship Jamboree in Boston. Our project Beadzillus finished 4th and won the prize for the "Best Wiki" (with Slovenia) and "Best New Application Project".
[ more news ]

Inducible Bacillus Promoters
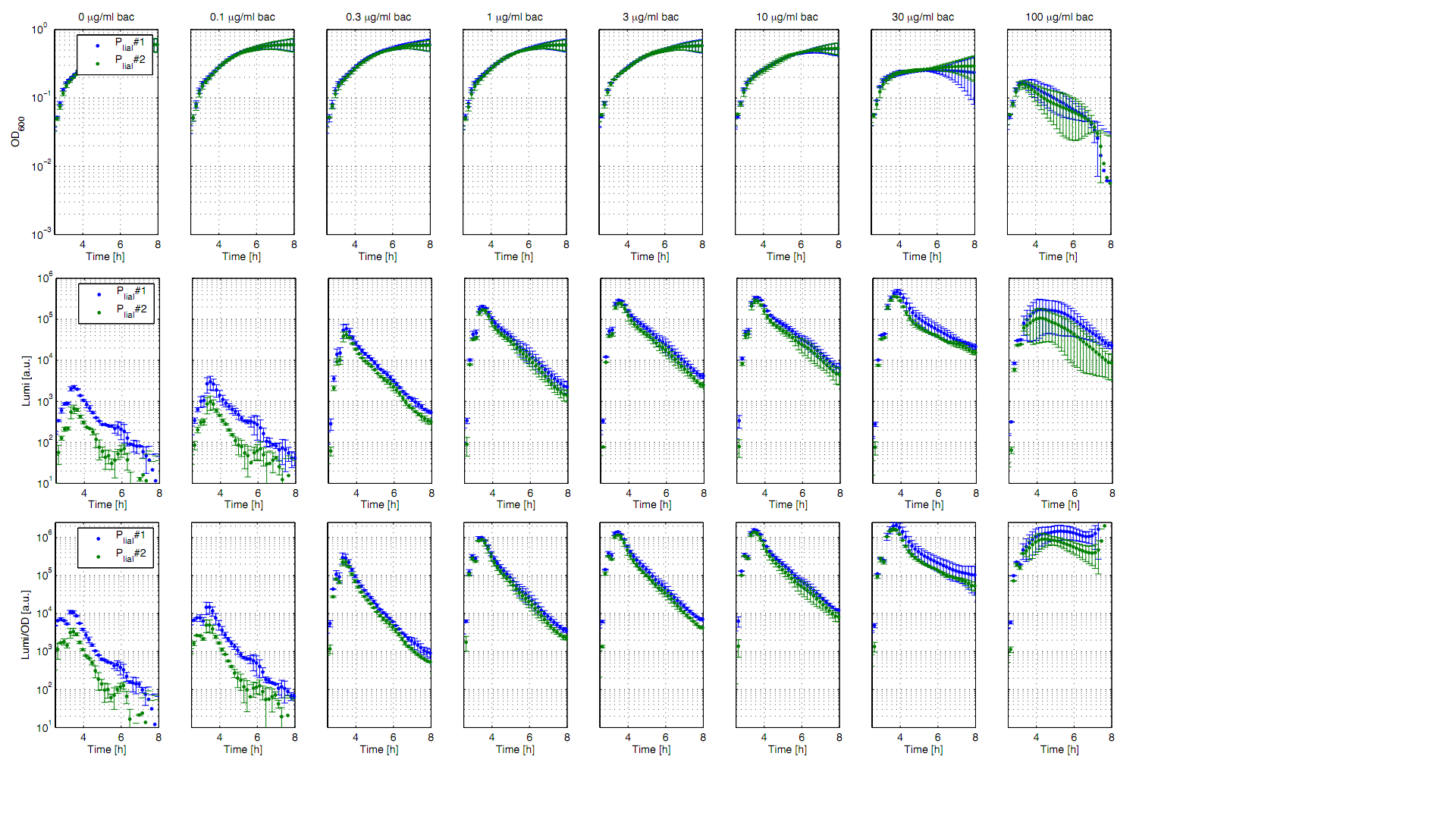
Fig. 1: Luminescence measurements of the inducible B. subtilis promoter PliaI after induction with different concentrations of bacitracin. OD600 (up), luminescence (middle), and luminescence/OD600 (down) of the two clones (green/blue) depending on the time (h) after induction with bacitracin (0 μg/ml(left) to 100μg/ml(right)). Data derive from three independent experiments, the graphs show the mean fo the three experiments and the standard deviation. Curves were fitted over each other (t=0, OD600=0,3) and smoothed by taking the average of three neighboring values. t=0 is the time of induction.
The inducible promoter PliaI was evaluated in the reporter vector pSBBs3C-luxABCDE which contains the lux operon ![]() . This is why the promoter activity leads to gene expression and to the production of the protein luciferase. The luminescence of this protein can be measured with the plate reader Synergy2 (Biotek) (Fig.1). All clones show a normal growth behaviour up to a bacitracin concentration of 10 μg/ml. At higher concentrations of bacitracin the growth curves decrease because the cells die in presence of bacitracin. The promoter PliaI shows a basal activity level of about 10.000 Lumi/OD600 at the maximum. After induction with bacitracin the luminescence per OD600 increases depending of the concentration of bacitracin. The highest activity of about 1.5 Mio Lumi/OD600 can be measured after induction with 10-30 μg/ml bacitracin. If the concentration gets higher than 100 μg/ml the luminescence of both clones show a different behaviour. To see also the induction of this inducible promoter in comparison to an uninducible promoter we also did induction experiments with PliaG which should not be inducible. As expected there is no response in activity depending on the induction and PliaG shows a constant maximum of activity of about 10.000 Lumi/OD600 (Data not shown).
. This is why the promoter activity leads to gene expression and to the production of the protein luciferase. The luminescence of this protein can be measured with the plate reader Synergy2 (Biotek) (Fig.1). All clones show a normal growth behaviour up to a bacitracin concentration of 10 μg/ml. At higher concentrations of bacitracin the growth curves decrease because the cells die in presence of bacitracin. The promoter PliaI shows a basal activity level of about 10.000 Lumi/OD600 at the maximum. After induction with bacitracin the luminescence per OD600 increases depending of the concentration of bacitracin. The highest activity of about 1.5 Mio Lumi/OD600 can be measured after induction with 10-30 μg/ml bacitracin. If the concentration gets higher than 100 μg/ml the luminescence of both clones show a different behaviour. To see also the induction of this inducible promoter in comparison to an uninducible promoter we also did induction experiments with PliaG which should not be inducible. As expected there is no response in activity depending on the induction and PliaG shows a constant maximum of activity of about 10.000 Lumi/OD600 (Data not shown).
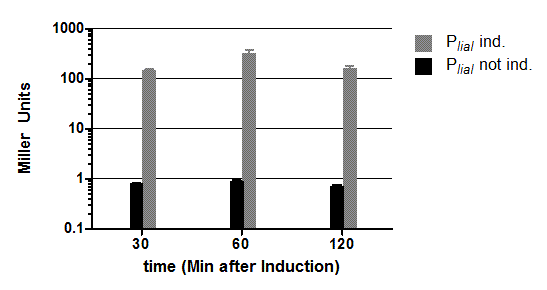
Fig. 2: β-galactosidase assay with (grey) and without (black) induction of bacitracin (20μg/ml) of strains carrying the promoter PliaI fused to lacZ. The average of the β-galactosidase activity (Miller Units) and the standard deviation of two independent clones are shown depending on the time(minutes after induction). Experiment shows representative data which was obtained in the same way from three independent experiments.
The inducible promoter PliaI was also evaluated with the reporter vector pSBBs1C-lacZ which contains the lacZ reporter gene ![]() (Fig.2). Promoter activity leads to the expression of the β-galactosidase which directly correlates to the promoter activity. The β-galactosidase assay of the inducible Bacillus promoter PliaI was repeated three times. Data show one representative result.
(Fig.2). Promoter activity leads to the expression of the β-galactosidase which directly correlates to the promoter activity. The β-galactosidase assay of the inducible Bacillus promoter PliaI was repeated three times. Data show one representative result.
In the beginning of the growth curve both promoters show only low activity. But then it increases to a maximum before it decreases to the begininng level after about seven hours (Data not shown). Summing up the course of activity of both promoters Pveg and PliaG is very similar based on the growth curve. The highest β-galactosidase activity and therefore the highest activity of the promoter Pveg with a maximum of 65 Miller units can be found during the transition from the logarithmic to the stationary phase. This is about five times higher than the acitivity of the promoter PliaG with a maximum activity of about 12 Miller Units. </p>
Der β-Galaktosidase Assay des induzierbaren B. subtilis-Promotors PliaI wurde wie in 2.5.4. beschrieben mit dem Stamm iGEMB13 mit und ohne Induktion durch Bacitracin (20μg/ml) bei einer OD600 von 0,4 durchgeführt. Dabei wird durch den entstandenen gelben Farbstoff auf die Enzymmenge an β-Galaktosidase und somit auf die Promotoraktivität, die für die Stärke der Expression verantwortlich ist, geschlossen. Die Ergebnisse einer Messung, die sich in drei unabhängigen Experimenten bestätigten, sind in Abb. 9 gezeigt. Der Promotor PliaI zeigt ohne Induktion durch Bacitracin keine Aktivität. Nach Induktion durch Bacitracin (20μg/ml) ist die Expression der β-Galaktosidase sehr stark, wodurch so viel Enzym entsteht, dass die gesamte Aktivität des Enzyms bereits nach 30 Minuten etwa 200 Miller Units erreicht, bevor sie auf ein Maximum von etwa 500 Miller Units ansteigt (t=60 Min). Somit ist die Aktivität des Enzyms und somit des Promotors nach Induktion mit dieser Bacitracin-Konzentration an ihrem Maximum etwa 500 Mal größer als ohne Induktion.
 "
"



