Team:LMU-Munich/Data/Anderson
From 2012.igem.org
| Line 3: | Line 3: | ||
===Anderson Promoters=== | ===Anderson Promoters=== | ||
| - | + | <br> | |
| - | + | <br> | |
[[File:Auswertung Anderson promoters.png|thumb|right|400px| <p align="justify"> '''Fig. 1: Luminescence measurement of Anderson promoters in the reporter vector pSB<sub>''Bs''</sub>3C-''luxABCDE'''''. OD<sub>''600''</sub> (right), LUMI (middle) and OD<sub>''600''</sub> per LUMI (left) depending on the time (h) are shown for two different clones (green/blue). Data derive from three independent experiments, so graph shows the mean with the standard deviation. Curves were fitted over each other (t=0, OD<sub>''600''</sub>=0,3) and smoothed by taking average of three neighboring values.</p>]] | [[File:Auswertung Anderson promoters.png|thumb|right|400px| <p align="justify"> '''Fig. 1: Luminescence measurement of Anderson promoters in the reporter vector pSB<sub>''Bs''</sub>3C-''luxABCDE'''''. OD<sub>''600''</sub> (right), LUMI (middle) and OD<sub>''600''</sub> per LUMI (left) depending on the time (h) are shown for two different clones (green/blue). Data derive from three independent experiments, so graph shows the mean with the standard deviation. Curves were fitted over each other (t=0, OD<sub>''600''</sub>=0,3) and smoothed by taking average of three neighboring values.</p>]] | ||
<p align="justify"> | <p align="justify"> | ||
| - | Eleven of the nineteen promoters of the [http://partsregistry.org/Part:BBa_J23100 '''Anderson collection'''] (J23100, J23101, J23102, J23103, J23106, J23107, J23113, J23114, J23115, J23117, J23118) were evaluated. Therefore we used the reporter vector pSB<sub>''Bs''</sub>3C-''luxABCDE'' from the BioBrickBox containing the ''lux'' operon as a reporter for promoter activity. The promoter activity leads to the expression of the ''lux'' operon and to the production of the enzyme luciferase. The luminescence which is produced by the luciferase can be measured with the plate reader ''Synergy2''(BioTek)('''Fig.1'''). All clones show a usual growth behaviour. The activity of the promoters increases during transition from log to stationary phase. This maximum (t=1h) reaches from 200Lumi/OD<sub>600</sub> (promoter J23115) to a maximum of 1500 Lumi/OD<sub>600</sub> for the strongest promoter (J23101). Afterwards the activity goes down to the beginning level (t=2h). The oscillation of luminescence (Lumi/ OD<sub>600</sub>) in the beginning of the curves are due to the small OD<sub>600</sub> values and do not mean a high promoter activity. One clone of J23107 and J23114 shows significantly lower promoter activity. Therefore additional clones should be measured. In comparison to all the other evaluated ''Bacillus'' promoters these Anderson promoters showed a very low acitivity in ''B. subtilis''. | + | Eleven of the nineteen promoters of the [http://partsregistry.org/Part:BBa_J23100 '''Anderson collection'''] (J23100, J23101, J23102, J23103, J23106, J23107, J23113, J23114, J23115, J23117, J23118) were evaluated. Therefore we used the reporter vector pSB<sub>''Bs''</sub>3C-''luxABCDE'' from the BioBrickBox containing the ''lux'' operon as a reporter for promoter activity. The promoter activity leads to the expression of the ''lux'' operon and to the production of the enzyme luciferase. The luminescence which is produced by the luciferase can be measured with the plate reader ''Synergy2'' (BioTek) ('''Fig.1'''). All clones show a usual growth behaviour. The activity of the promoters increases during transition from log to stationary phase. This maximum (t=1h) reaches from 200Lumi/OD<sub>600</sub> (promoter J23115) to a maximum of 1500 Lumi/OD<sub>600</sub> for the strongest promoter (J23101). Afterwards the activity goes down to the beginning level (t=2h). The oscillation of luminescence (Lumi/ OD<sub>600</sub>) in the beginning of the curves are due to the small OD<sub>600</sub> values and do not mean a high promoter activity. One clone of J23107 and J23114 shows significantly lower promoter activity. Therefore additional clones should be measured. In comparison to all the other evaluated ''Bacillus'' promoters these Anderson promoters showed a very low acitivity in ''B. subtilis''. |
<br> | <br> | ||
<br> | <br> | ||
Revision as of 12:43, 21 September 2012

The LMU-Munich team is exuberantly happy about the great success at the World Championship Jamboree in Boston. Our project Beadzillus finished 4th and won the prize for the "Best Wiki" (with Slovenia) and "Best New Application Project".
[ more news ]

Anderson Promoters
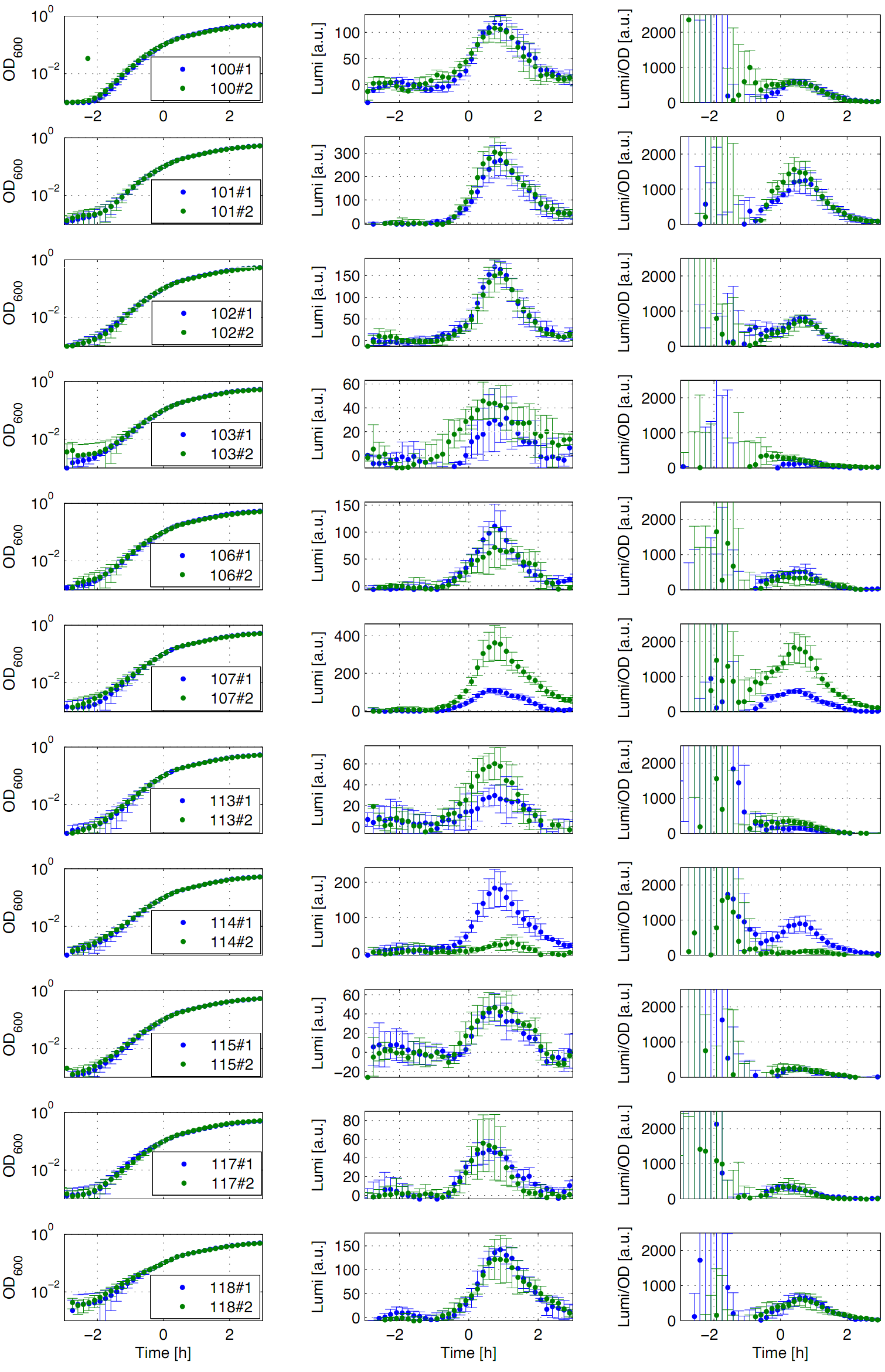
Fig. 1: Luminescence measurement of Anderson promoters in the reporter vector pSBBs3C-luxABCDE. OD600 (right), LUMI (middle) and OD600 per LUMI (left) depending on the time (h) are shown for two different clones (green/blue). Data derive from three independent experiments, so graph shows the mean with the standard deviation. Curves were fitted over each other (t=0, OD600=0,3) and smoothed by taking average of three neighboring values.
Eleven of the nineteen promoters of the [http://partsregistry.org/Part:BBa_J23100 Anderson collection] (J23100, J23101, J23102, J23103, J23106, J23107, J23113, J23114, J23115, J23117, J23118) were evaluated. Therefore we used the reporter vector pSBBs3C-luxABCDE from the BioBrickBox containing the lux operon as a reporter for promoter activity. The promoter activity leads to the expression of the lux operon and to the production of the enzyme luciferase. The luminescence which is produced by the luciferase can be measured with the plate reader Synergy2 (BioTek) (Fig.1). All clones show a usual growth behaviour. The activity of the promoters increases during transition from log to stationary phase. This maximum (t=1h) reaches from 200Lumi/OD600 (promoter J23115) to a maximum of 1500 Lumi/OD600 for the strongest promoter (J23101). Afterwards the activity goes down to the beginning level (t=2h). The oscillation of luminescence (Lumi/ OD600) in the beginning of the curves are due to the small OD600 values and do not mean a high promoter activity. One clone of J23107 and J23114 shows significantly lower promoter activity. Therefore additional clones should be measured. In comparison to all the other evaluated Bacillus promoters these Anderson promoters showed a very low acitivity in B. subtilis.
To measure the activity not only with the lux reporter operon, four promoters of the Anderson collection were cloned into the reporter vector pSBBs1C-lacZ to do β-galactosidase assays and then to compare the results of the strength of these promoters in B. subtilis. (Fig. 2)
 "
"



