Team:Bielefeld-Germany/Labjournal/week19
From 2012.igem.org
(Difference between revisions)
(→Monday September 3rd) |
(→Monday September 3rd) |
||
| Line 5: | Line 5: | ||
===Monday September 3rd=== | ===Monday September 3rd=== | ||
| + | [[File:Bielefeld2012_B_pumi_03_09.png|thumb|right|200px|Activity in oxidizing ABTS of our produced ''B. pumi'' laccase depending on time. Values are calculated by taking the average out of 4 measurements (n=4).]] | ||
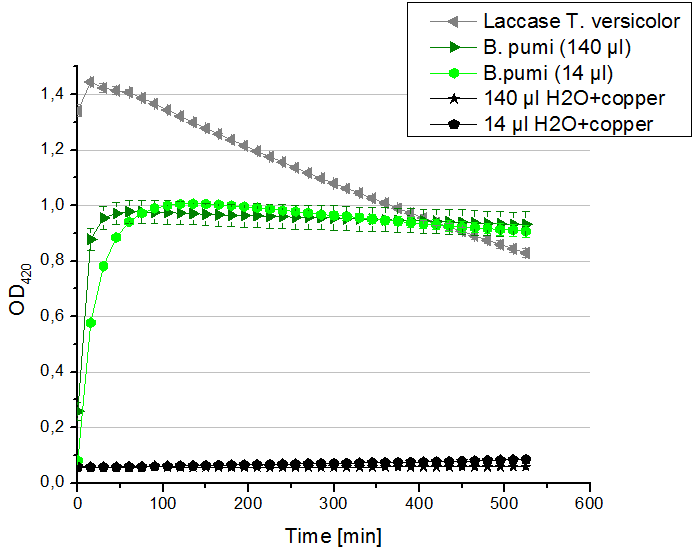
* '''Team Activity Tests:''' Today our hard-working Team Cultivation offered us some laccases from ''B. pumi''. After fermentation and TALON purification the laccase was still hanging around in imidazole buffer, so we rebuffered it into nanopure water. We incubated the sample with copper and considered to measure the protein concentration before running the activity tests. Using the protocol for determining the protein concentration via Bradford (standards were 1 mg/ml, 1.5 mg/ml and 2 mg/ml BSA) we got a final ''B. pumi'' laccase concentration of 0.22 mg/ml. With this information we designed the following activity test different as usual. As a positive control we took 140 µl of our bought laccase from ''T. versicolor'' with a concentration of 0,021 mg/ml. Accordingly to this concentration we applied 14 µl of the ''B. pumi'' laccase to get approximately the same amount of laccase into each well. Additionally we tested 140 µl of the ''B. pumi'' laccase to make sure that we will definitely see any activity. After measuring the samples the whole night we got our results in the morning. It turned out, that our store bought laccase was rapidly active as seen before in our activity tests. The ''B. pumi'' laccase was also active. Using 140 µl of it led to the maximal state of oxidation of ABTS after 45 minutes. With the reduced amount of 14 µl of laccase this optimum was reached after 90 minutes. For our following activity tests we want to include the protein concentration again. | * '''Team Activity Tests:''' Today our hard-working Team Cultivation offered us some laccases from ''B. pumi''. After fermentation and TALON purification the laccase was still hanging around in imidazole buffer, so we rebuffered it into nanopure water. We incubated the sample with copper and considered to measure the protein concentration before running the activity tests. Using the protocol for determining the protein concentration via Bradford (standards were 1 mg/ml, 1.5 mg/ml and 2 mg/ml BSA) we got a final ''B. pumi'' laccase concentration of 0.22 mg/ml. With this information we designed the following activity test different as usual. As a positive control we took 140 µl of our bought laccase from ''T. versicolor'' with a concentration of 0,021 mg/ml. Accordingly to this concentration we applied 14 µl of the ''B. pumi'' laccase to get approximately the same amount of laccase into each well. Additionally we tested 140 µl of the ''B. pumi'' laccase to make sure that we will definitely see any activity. After measuring the samples the whole night we got our results in the morning. It turned out, that our store bought laccase was rapidly active as seen before in our activity tests. The ''B. pumi'' laccase was also active. Using 140 µl of it led to the maximal state of oxidation of ABTS after 45 minutes. With the reduced amount of 14 µl of laccase this optimum was reached after 90 minutes. For our following activity tests we want to include the protein concentration again. | ||
Revision as of 09:24, 14 September 2012
Contents |
Week 19 (09/03 - 09/09/12)
Monday September 3rd
- Team Activity Tests: Today our hard-working Team Cultivation offered us some laccases from B. pumi. After fermentation and TALON purification the laccase was still hanging around in imidazole buffer, so we rebuffered it into nanopure water. We incubated the sample with copper and considered to measure the protein concentration before running the activity tests. Using the protocol for determining the protein concentration via Bradford (standards were 1 mg/ml, 1.5 mg/ml and 2 mg/ml BSA) we got a final B. pumi laccase concentration of 0.22 mg/ml. With this information we designed the following activity test different as usual. As a positive control we took 140 µl of our bought laccase from T. versicolor with a concentration of 0,021 mg/ml. Accordingly to this concentration we applied 14 µl of the B. pumi laccase to get approximately the same amount of laccase into each well. Additionally we tested 140 µl of the B. pumi laccase to make sure that we will definitely see any activity. After measuring the samples the whole night we got our results in the morning. It turned out, that our store bought laccase was rapidly active as seen before in our activity tests. The B. pumi laccase was also active. Using 140 µl of it led to the maximal state of oxidation of ABTS after 45 minutes. With the reduced amount of 14 µl of laccase this optimum was reached after 90 minutes. For our following activity tests we want to include the protein concentration again.
Tuesday September 4th
- We signed in for our tracks today! Our first choice is the 'Environment' track since it is obvious that we want to clean up drinking water and therefore help our environment. The second choice is the 'New Application' track. We are hoping our filter system can be applied in wastewater treatment plants and establishes a new area of water purification.
Wednesday September 5th
Thursday September 6th
Friday September 7th
Saturday September 8th
Sunday September 9th
Sunday
| 55px | | | | | | | | | | |
 "
"