Team:NRP-UEA-Norwich/Experiments
From 2012.igem.org
(→Test of the effect transforming E.coli with construct 1 and construct 2 has on the rate of growth) |
|||
| Line 10: | Line 10: | ||
The study involved testing the affects of transforming E.coli with different promoters on its growth over time. The promoters E.coli had been transformed with were PyeaR, M-B and B-M. These are promoters which all react to nitrogenous species. By running these together, we can obtain a direct comparison between all three of these promoters on the growth of E.coli. To see if there are any significant changes, the study was run alongside E.oli cells which had not been transformed with anything. For the rest of this brief report, untransformed cells will be referred to as Alpha cells and the other E.coli cells with transformations will be referred to as the promoter with which they were transformed with. | The study involved testing the affects of transforming E.coli with different promoters on its growth over time. The promoters E.coli had been transformed with were PyeaR, M-B and B-M. These are promoters which all react to nitrogenous species. By running these together, we can obtain a direct comparison between all three of these promoters on the growth of E.coli. To see if there are any significant changes, the study was run alongside E.oli cells which had not been transformed with anything. For the rest of this brief report, untransformed cells will be referred to as Alpha cells and the other E.coli cells with transformations will be referred to as the promoter with which they were transformed with. | ||
| + | |||
The E.coli cells used in the study and for the transformation are the same type of cells (Alpha select gold standard cells from Bioline). A colony was inoculated into 5ml of LB media overnight and the cells spun down the following morning and diluted with fresh LB until an OD reading at 600nm of 0.2 ± 0.01 was obtained. Three repeats were made of each sample. | The E.coli cells used in the study and for the transformation are the same type of cells (Alpha select gold standard cells from Bioline). A colony was inoculated into 5ml of LB media overnight and the cells spun down the following morning and diluted with fresh LB until an OD reading at 600nm of 0.2 ± 0.01 was obtained. Three repeats were made of each sample. | ||
| + | |||
The study lasted for 12 hours. An OD reading at 600nm was taken once an hour. Between the hour, the cuvettes were put into a 37ᵒC incubator to encourage growth and for standardising measurements with other growth studies. | The study lasted for 12 hours. An OD reading at 600nm was taken once an hour. Between the hour, the cuvettes were put into a 37ᵒC incubator to encourage growth and for standardising measurements with other growth studies. | ||
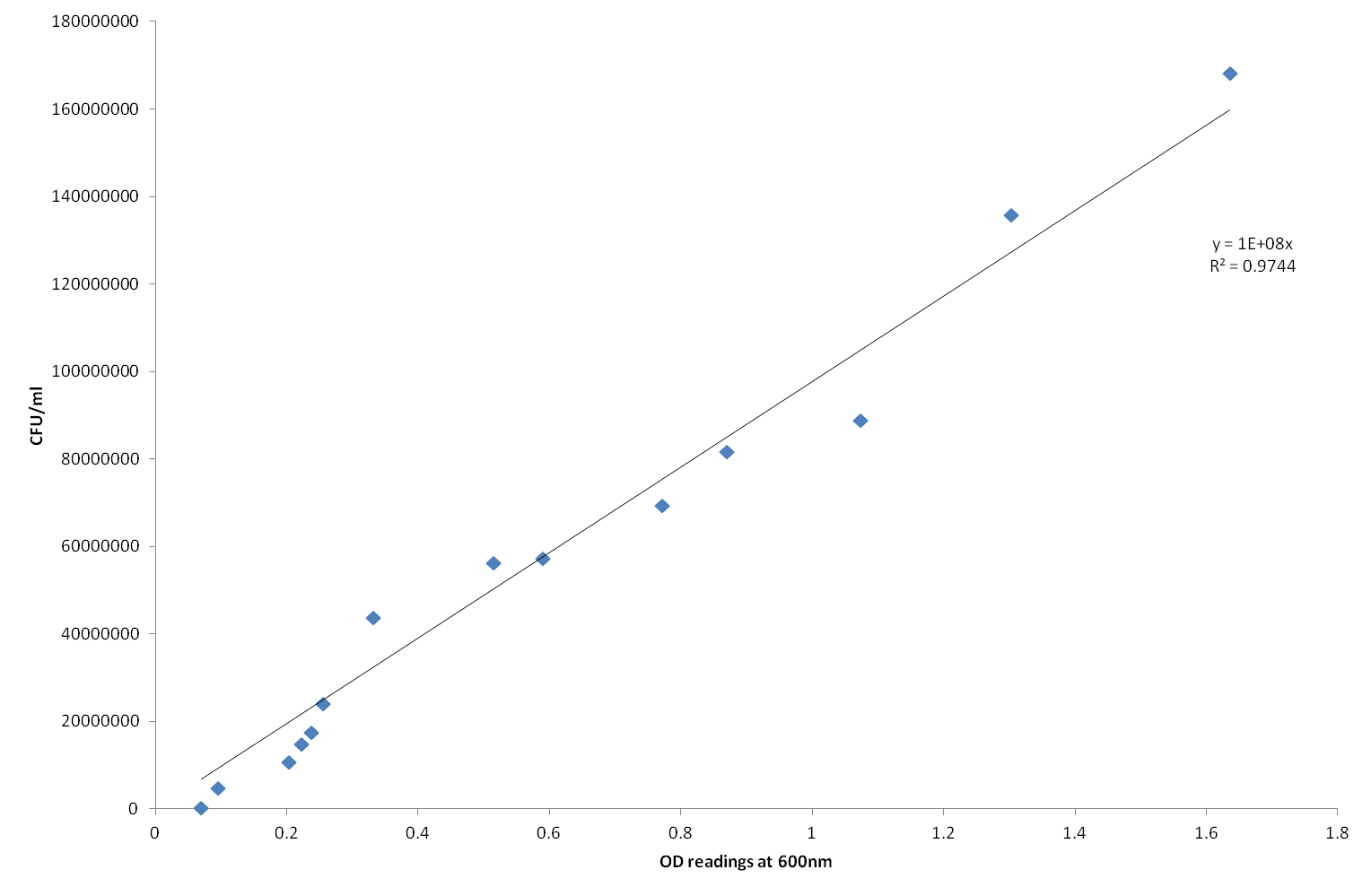
| - | To calculate the number of cells in the samples, a calibration curve was set up. This involved using cultures of the E.coli cells without transformations. The E.coli cells were diluted with different volumes of LB and OD readings were taken as well as plating on Agar plates. After a day of growth, the numbers on these plates were counted and recorded. The CFU/ml was calculated. When the OD readings (x axis) and the CFU/ml (y axis) readings are plotted, the equation of the line of best fit, gives a conversion for the absorbance readings. This allowed us to measure the growth. | + | To calculate the number of cells in the samples, a calibration curve was set up. This involved using cultures of the E.coli cells without transformations. The E.coli cells were diluted with different volumes of LB and OD readings were taken as well as plating on Agar plates. After a day of growth, the numbers on these plates were counted and recorded. The CFU/ml was calculated. When the OD readings (x axis) and the CFU/ml (y axis) readings are plotted, the equation of the line of best fit, gives a conversion for the absorbance readings. This allowed us to measure the growth. This is demonstrated in figure 1. |
| + | |||
| + | [[File:Calibration curve.png | 500px | center]] | ||
| + | Figure 1: Calibration curve to calculate the conversion factor between OD reading at 600nm and the number of colony forming units growing per ml (CFU/ml) | ||
| + | |||
We found that there was a significant difference between Alpha cells and PyeaR cells. Initially, Alpha cells had a greater growth rate, but after the third hour into the study, the growth rate of PyeaR was faster than that of Alpha cells. The overall growth rate of PyeaR cells was significantly faster that Alpha cells (Levenes Test, F = 1.009 p = 0.372; T Test, t = 4.196, df = 4, p = 0.014). | We found that there was a significant difference between Alpha cells and PyeaR cells. Initially, Alpha cells had a greater growth rate, but after the third hour into the study, the growth rate of PyeaR was faster than that of Alpha cells. The overall growth rate of PyeaR cells was significantly faster that Alpha cells (Levenes Test, F = 1.009 p = 0.372; T Test, t = 4.196, df = 4, p = 0.014). | ||
| Line 19: | Line 25: | ||
| - | Figure | + | Figure 2: Growth of PyeaR transformed E.coli cells relative to Alpha cell (untransformed cells. Error bars show the standard deviation between the three repeats. For clarity reasons, lines of best fit are not shown |
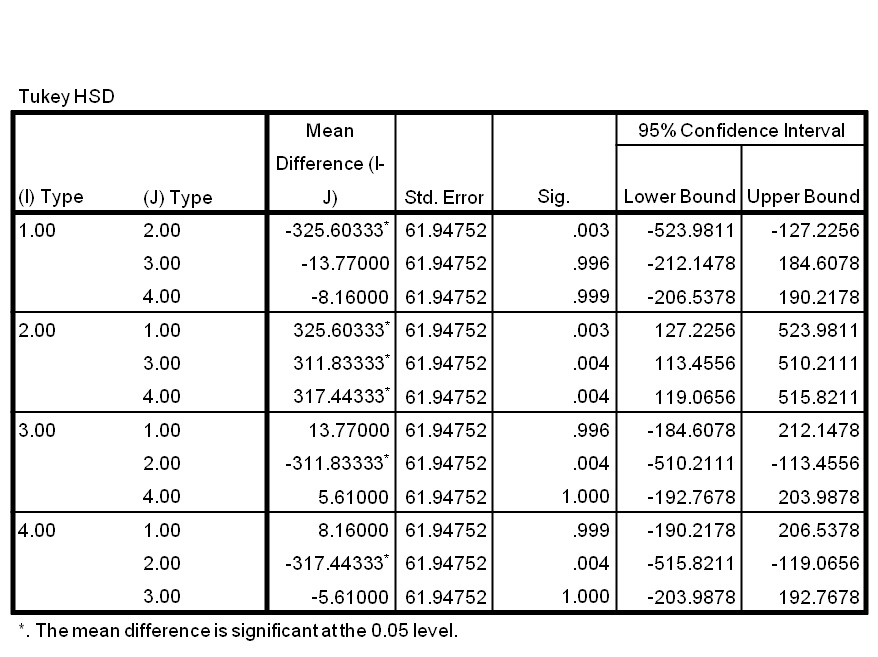
The growth pattern and rate of E.coli cells with or without transformation with B-M and M-B show little difference. Any differences in growth rate were not significant. There was lots of overlap. As previously described, there was a significant difference between the growth rate of PyeaR and Alpha cells. There was also a significant difference between MB/BM and PyeaR cells. The statistical results can be seen in Table 1 | The growth pattern and rate of E.coli cells with or without transformation with B-M and M-B show little difference. Any differences in growth rate were not significant. There was lots of overlap. As previously described, there was a significant difference between the growth rate of PyeaR and Alpha cells. There was also a significant difference between MB/BM and PyeaR cells. The statistical results can be seen in Table 1 | ||
[[File:A+M+B.png | 500px | center]] | [[File:A+M+B.png | 500px | center]] | ||
| - | Figure | + | Figure 3: Growth over 12 hours of Alpha, M-B and B-M. Error bars and lines of best fit are not shown for clarity reasons. |
Revision as of 20:38, 11 September 2012
 "
"