User:DrJones1935/22 July 2012
From 2012.igem.org
(Difference between revisions)
(Created page with "====I. PCR==== *'''Mix Reagents:''' :*Mix according to the following table, where 2 = LacZ, 3 = CAT*, 4 = tet<sup>r</sup>, 0.1-0.3 = +template, and 0.4 = -template ::*For exampl...") |
(→I. PCR) |
||
| Line 1: | Line 1: | ||
| - | ====I. PCR==== | + | ====I. Re-run AGE on PCR samples==== |
| + | |||
| + | *''SAME RESULTS AS ABOVE'' | ||
| + | |||
| + | ====II. Re-run PCR of questionable samples==== | ||
| + | |||
*'''Mix Reagents:''' | *'''Mix Reagents:''' | ||
| - | :*Mix according to the following table, where 2 = LacZ, 3 = CAT* | + | :*Mix according to the following table, where 2 = LacZ, 3 = CAT*, 0.1-0.3 = +template, and 0.4 = -template |
::*For example, 2.1 = lacZ sample, 2.4 = lacZ control | ::*For example, 2.1 = lacZ sample, 2.4 = lacZ control | ||
{| class="wikitable" | {| class="wikitable" | ||
|- | |- | ||
| - | ! !! 2.1 !! 2.2 !! 2.3 !! 2.4 !! 3.1 !! 3.2 !! 3.3 !! 3 | + | ! !! 2.1 !! 2.2 !! 2.3 !! 2.4 !! 3.1 !! 3.2 !! 3.3 !! 3.4 |
|- | |- | ||
| - | ! Reagent | + | ! Reagent !! (uL) !! (uL) !! (uL) !! (uL) !! (uL) !! (uL) !! (uL) !! (uL) |
|- | |- | ||
| - | | water || 33.5 || 33.5 || 33.5 || 33.5 || 33.5 || 33.5 || 33 | + | | water || 33.5 || 33.5 || 33.5 || 33.5 || 33.5 || 33.5 || 33.5 || 33.5 |
|- | |- | ||
| - | | 5x Phusion HiFi buffer | + | | 5x Phusion HiFi buffer || 10 || 10 || 10 || 10 || 10 || 10 || 10 || 10 |
|- | |- | ||
| - | | 10 mM dNTP mix | + | | 10 mM dNTP mix || 1 || 1 || 1 || 1 || 1 || 1 || 1 || 1 |
|- | |- | ||
| - | | 10 uM primer (F) || 2.5 (p2f_2) || 2.5 (p2f_2 || 2.5 (p2f_2) || 2.5 (p2f_2) || 2.5 (p3f_2) || 2.5 (p3f_2) || 2.5 (p3f_2) || 2.5 (p3f_2 | + | | 10 uM primer (F) || 2.5 (p2f_2) || 2.5 (p2f_2 || 2.5 (p2f_2) || 2.5 (p2f_2) || 2.5 (p3f_2) || 2.5 (p3f_2) || 2.5 (p3f_2) || 2.5 (p3f_2) |
|- | |- | ||
| - | | 10 uM primer (R) || 2.5 (p2r_2) || 2.5 (p2r_2) || 2.5 (p2r_2) || 2.5 (p2r_2) || 2.5 (p3r) || 2.5 (p3r) || 2.5 (p3r) || 2.5 (p3r | + | | 10 uM primer (R) || 2.5 (p2r_2) || 2.5 (p2r_2) || 2.5 (p2r_2) || 2.5 (p2r_2) || 2.5 (p3r) || 2.5 (p3r) || 2.5 (p3r) || 2.5 (p3r) |
|- | |- | ||
| - | | Template || ECNR2 colony* || ECNR2 colony* || ECNR2 colony* || 0 || ECFI5 colony* || ECFI5 colony* || ECFI5 colony* | + | | Template || ECNR2 colony* || ECNR2 colony* || ECNR2 colony* || 0 || ECFI5 colony* || ECFI5 colony* || ECFI5 colony* || 0 |
|- | |- | ||
| - | | Phusion HiFi polymerase | + | | Phusion HiFi polymerase || 0.5 || 0.5 || 0.5 || 0.5 || 0.5 || 0.5 || 0.5 || 0.5 |
|} | |} | ||
| Line 57: | Line 62: | ||
|} | |} | ||
*Store tubes on ice after PCR is finished | *Store tubes on ice after PCR is finished | ||
| + | |||
| + | ====III. Agarose Gel Electrophoresis (AGE) of PCR Samples==== | ||
| + | |||
| + | *'''Make Gel:''' | ||
| + | :*Measure out <strike>0.5 g</strike> 0.50231 g of agarose and add to a 250 mL E-flask | ||
| + | :*Add 50 mL of 0.5x TBE buffer and swirl to mix | ||
| + | :*Cover flask with a Kimwipe and microwave for ~1 minute until clear | ||
| + | :*Allow to cool to ~60 <sup>o</sup>C and pour into beaker for ethidium bromide (EtBr) | ||
| + | :*Add 1 uL of EtBr stock to agarose and swirl to mix | ||
| + | :*Immediately pour gel into tray with combs | ||
| + | :*Allow to solidify, then remove the comb and tape and place the tray in the gel box with the wells closer to the black electrode | ||
| + | ::*Make sure it is submerged in TBE buffer | ||
| + | |||
| + | *'''Load Gel:''' | ||
| + | :*Mix samples as drops on a piece of parafilm | ||
| + | ::*Mix: | ||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | ! Ladder (Invtrogen 1 kb plus) !! Blank !! Sample | ||
| + | |- | ||
| + | | *5 uL DNA ladder<br>*1 uL 6x Loading Dye || *8.3 uL dH<sub>2</sub>O<br>*1.7 uL 6x Loading Dye || *6.3 uL dH<sub>2</sub>O<br>*1.7 uL 6x Loading Dye<br>*2.0 uL DNA | ||
| + | |} | ||
| + | :*Load ~6 uL on gel according to the following chart: | ||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | ! Lane 1 !! 2 !! 3 !! 4 !! 5 !! 6 !! 7 !! 8 !! 9 !! 10 !! 11 | ||
| + | |- | ||
| + | | NEB 2-log ladder || Blank || 2.1 || 2.2 || 2.3 || 2.4 || 3.1 || 3.2 || 3.3 || 3.4 | ||
| + | |} | ||
| + | :*Store DNA at 4 <sup>o</sup>C | ||
| + | |||
| + | *'''Run Gel:''' | ||
| + | :*Close gel box and turn on power pack | ||
| + | :*Run gel at 30 V for 20 min to stack gel | ||
| + | :*Run gel at 75 V until the markers have reached ~halfway down the well | ||
| + | |||
| + | *'''Image Gel:''' | ||
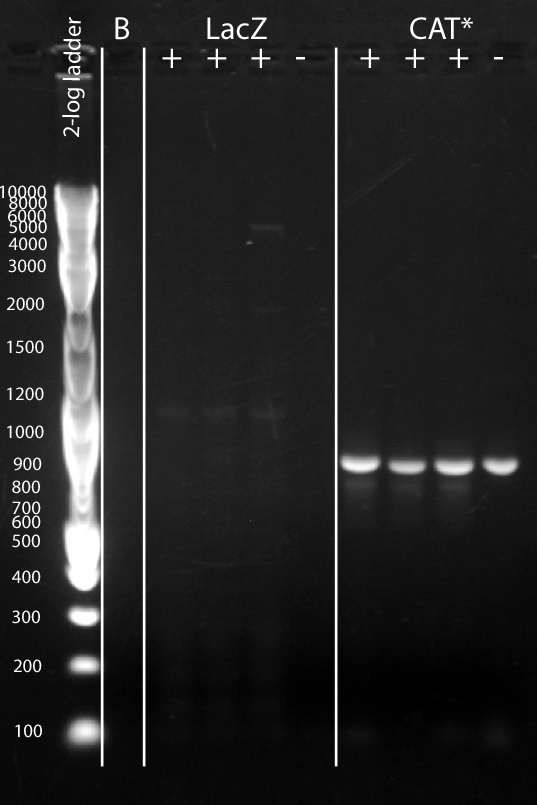
| + | :*''RESULTS:'' | ||
| + | [[File:Srk_2012-07-22_19hr_22min.jpg|center|500px]] | ||
| + | Source: [[Media:Srk_2012-07-22_19hr_22min.tif]] | ||
| + | {| class="wikitable" | ||
| + | |- | ||
| + | ! Feature !! Expected Size (bp) | ||
| + | |- | ||
| + | | LacZ || 3151 | ||
| + | |- | ||
| + | | CAT* || 729 | ||
| + | |} | ||
| + | <br> | ||
| + | <br> | ||
| + | |||
| + | ---- | ||
====II. Agarose Gel Electrophoresis (AGE) of PCR Samples==== | ====II. Agarose Gel Electrophoresis (AGE) of PCR Samples==== | ||
Latest revision as of 23:25, 3 October 2012
Contents |
I. Re-run AGE on PCR samples
- SAME RESULTS AS ABOVE
II. Re-run PCR of questionable samples
- Mix Reagents:
- Mix according to the following table, where 2 = LacZ, 3 = CAT*, 0.1-0.3 = +template, and 0.4 = -template
- For example, 2.1 = lacZ sample, 2.4 = lacZ control
| 2.1 | 2.2 | 2.3 | 2.4 | 3.1 | 3.2 | 3.3 | 3.4 | |
|---|---|---|---|---|---|---|---|---|
| Reagent | (uL) | (uL) | (uL) | (uL) | (uL) | (uL) | (uL) | (uL) |
| water | 33.5 | 33.5 | 33.5 | 33.5 | 33.5 | 33.5 | 33.5 | 33.5 |
| 5x Phusion HiFi buffer | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| 10 mM dNTP mix | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 10 uM primer (F) | 2.5 (p2f_2) | 2.5 (p2f_2 | 2.5 (p2f_2) | 2.5 (p2f_2) | 2.5 (p3f_2) | 2.5 (p3f_2) | 2.5 (p3f_2) | 2.5 (p3f_2) |
| 10 uM primer (R) | 2.5 (p2r_2) | 2.5 (p2r_2) | 2.5 (p2r_2) | 2.5 (p2r_2) | 2.5 (p3r) | 2.5 (p3r) | 2.5 (p3r) | 2.5 (p3r) |
| Template | ECNR2 colony* | ECNR2 colony* | ECNR2 colony* | 0 | ECFI5 colony* | ECFI5 colony* | ECFI5 colony* | 0 |
| Phusion HiFi polymerase | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
*For these templates, I touched a colony with a sterile pipette tip and spread it on the bottom of the PCR tube before adding reagents
- Run PCR
| Step | Temp (oC) | Time |
|---|---|---|
| 1 | 94 | 4 m |
| 2 | 94 | 30 s |
| 3 | 53 | 30 s |
| 4 | 72 | 1.5 m |
| 5 | GOTO 2 | 7x |
| 6 | 94 | 30 s |
| 7 | 66 | 30 s |
| 8 | 72 | 1.5 m |
| 9 | GOTO 6 | 30x |
| 10 | 72 | 5 m |
| 11 | 4 | ∞* |
- Store tubes on ice after PCR is finished
III. Agarose Gel Electrophoresis (AGE) of PCR Samples
- Make Gel:
- Measure out
0.5 g0.50231 g of agarose and add to a 250 mL E-flask - Add 50 mL of 0.5x TBE buffer and swirl to mix
- Cover flask with a Kimwipe and microwave for ~1 minute until clear
- Allow to cool to ~60 oC and pour into beaker for ethidium bromide (EtBr)
- Add 1 uL of EtBr stock to agarose and swirl to mix
- Immediately pour gel into tray with combs
- Allow to solidify, then remove the comb and tape and place the tray in the gel box with the wells closer to the black electrode
- Make sure it is submerged in TBE buffer
- Measure out
- Load Gel:
- Mix samples as drops on a piece of parafilm
- Mix:
| Ladder (Invtrogen 1 kb plus) | Blank | Sample |
|---|---|---|
| *5 uL DNA ladder *1 uL 6x Loading Dye | *8.3 uL dH2O *1.7 uL 6x Loading Dye | *6.3 uL dH2O *1.7 uL 6x Loading Dye *2.0 uL DNA |
- Load ~6 uL on gel according to the following chart:
| Lane 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|
| NEB 2-log ladder | Blank | 2.1 | 2.2 | 2.3 | 2.4 | 3.1 | 3.2 | 3.3 | 3.4 |
- Store DNA at 4 oC
- Run Gel:
- Close gel box and turn on power pack
- Run gel at 30 V for 20 min to stack gel
- Run gel at 75 V until the markers have reached ~halfway down the well
- Image Gel:
- RESULTS:
Source: Media:Srk_2012-07-22_19hr_22min.tif
| Feature | Expected Size (bp) |
|---|---|
| LacZ | 3151 |
| CAT* | 729 |
II. Agarose Gel Electrophoresis (AGE) of PCR Samples
- Make Gel:
- Measure out
0.5 g0.50238 g of agarose and add to a 250 mL E-flask - Add 50 mL of 0.5x TBE buffer and swirl to mix
- Cover flask with a Kimwipe and microwave for ~1 minute until clear
- Allow to cool to ~60 oC and pour into beaker for ethidium bromide (EtBr)
- Add 1 uL of EtBr stock to agarose and swirl to mix
- Immediately pour gel into tray with combs
- Allow to solidify, then remove the comb and tape and place the tray in the gel box with the wells closer to the black electrode
- Make sure it is submerged in TBE buffer
- Measure out
- Load Gel:
- Mix samples as drops on a piece of parafilm
- Mix:
| Ladder (Invtrogen 1 kb plus) | Blank | Sample |
|---|---|---|
| *5 uL DNA ladder *1 uL 6x Loading Dye | *8.3 uL dH2O *1.7 uL 6x Loading Dye | *6.3 uL dH2O *1.7 uL 6x Loading Dye *2.0 uL DNA |
- Load ~10 uL (except ladder which is ~6 uL) on gel according to the following chart:
| Lane 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NEB 2-log ladder | Blank | 2.1 | 2.2 | 2.3 | 2.4 | 3.1 | 3.2 | 3.3 | 3.4 | 4.1 | 4.2 | 4.3 | 4.4 | NEB 2-log ladder |
- Store DNA at 4 oC
- Run Gel:
- Close gel box and turn on power pack
- Run gel at 30 V for 20 min to stack gel
- Run gel at 75 V until the markers have reached near the bottom of the gel
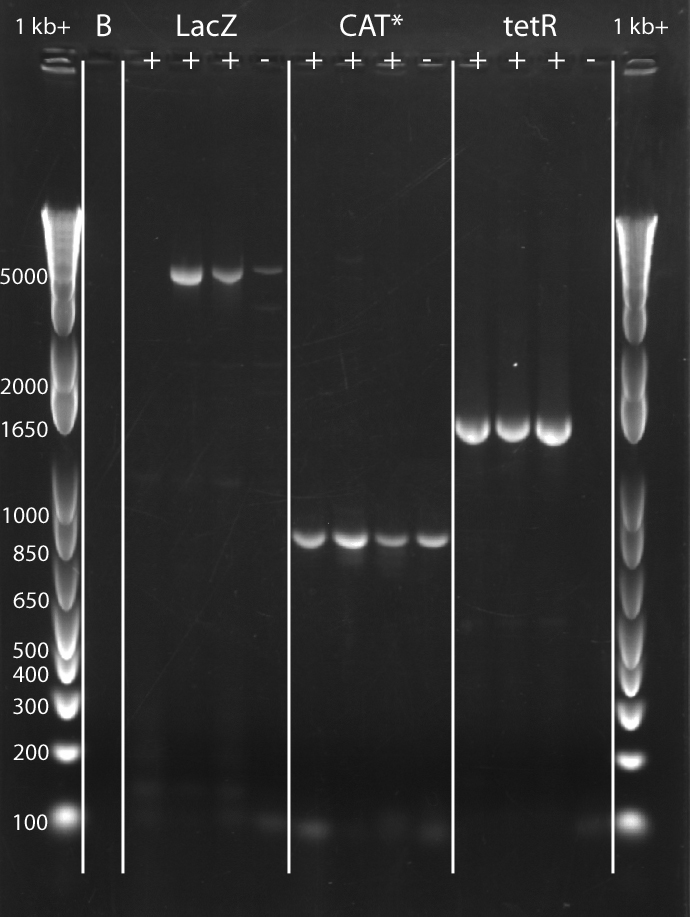
- Image Gel:
- RESULTS:
Source: Media:Srk_2012-07-21_17hr_37min.tif
| Feature | Expected Size (bp) |
|---|---|
| Leftover pBR322 | 4361 |
| LacZ | 3151 |
| CAT* | 729 |
| tetr | 1265 |
 "
"