Team:Washington/Safety
From 2012.igem.org
| Home | Team | Official Team Profile | Project | Parts Submitted to the Registry | Modeling | Notebook | Safety | Attributions |
|---|
Resources
People

- Markus Schmidt
- Biosafety Working Group, IDC, Austria
IDC`s Biosafety Working Group is active in the field of management and use of plant genetic resources, risk assessment of new biotechnologies, and safety and security issues of synthetic biology.
Reports
 Schmidt M. 2009. Do I understand what I can create? Biosafety issues in synthetic biology. Chapter 6 in: Schmidt M. Kelle A. Ganguli A, de Vriend H. (Eds.) 2009. Synthetic Biology. The Technoscience and its Societal Consequences. Springer Academic Publishing
Schmidt M. 2009. Do I understand what I can create? Biosafety issues in synthetic biology. Chapter 6 in: Schmidt M. Kelle A. Ganguli A, de Vriend H. (Eds.) 2009. Synthetic Biology. The Technoscience and its Societal Consequences. Springer Academic Publishing
 HSE 2009. The SACGM Compendium of guidance Part 2: Risk assessment of genetically modified microorganisms
HSE 2009. The SACGM Compendium of guidance Part 2: Risk assessment of genetically modified microorganisms
 Tucker JB and Zilinska,s RA. 2006. The Promise and Perils of Synthetic Biology . The new Atlantis. Spring 2006, p.25-45
Tucker JB and Zilinska,s RA. 2006. The Promise and Perils of Synthetic Biology . The new Atlantis. Spring 2006, p.25-45
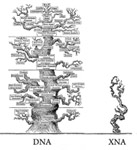 Schmidt M. 2010. Xenobiology: a new form of life as the ultimate biosafety tool . Bioessays 32:322-331
Schmidt M. 2010. Xenobiology: a new form of life as the ultimate biosafety tool . Bioessays 32:322-331
 Schmidt M, 2008. Diffusion of synthetic biology: a challenge to biosafety. Systems and Synthetic Biology. Vol.2(1-2):1-6
Schmidt M, 2008. Diffusion of synthetic biology: a challenge to biosafety. Systems and Synthetic Biology. Vol.2(1-2):1-6
 |
 |
 |
 |
 |
Safety
1. Would any of your project ideas raise safety issues in terms of: researcher safety, public safety, or environmental safety?
All projects are being conducted in lab-safe strains of E. coli or S. cerevisiae. All researchers have been trained in applicable lab safety to insure that no bacteria are inadvertently released into the environment. The researchers have also been trained in proper handling of chemicals. In both the influenza and plastics projects, the actual organisms being engineered were maintained in lab conditions (cultures, bioreactors, etc.). For the flu binder project, no live or attenuated influenza virus was used in any way. We did not handle, culture, transport, or otherwise come into contact with influenza virus, nor do we plan to. Our flu binders are against the surface protein hemagglutinin, which we obtained in the purified form from the Wilson lab at Scripps. Binding proteins were tested only against the recombinantly expressed and purified ectodomain of the hemagglutinin protein, and never against any form of the virus itself. Hemaggluinin is a non-toxic protein, and has no catalytic properties.
2. Do any of the new BioBrick parts (or devices) that you made this year raise any safety issues?
None of the parts we made this year raise any particular safety issues that we can foresee. All of our major parts were either taken from pre-registered biobricks, obtained from XXXXX in DNA form, cloned from non-pathogenic standard lab strains of E. Coli or synthesized from scratch using oligos and gBlocks ordered from IDT (http://www.idtdna.com/). None of our new parts would provide any foreseeable selective advantage in the wild. Thus, these parts would not increase bacterial survival in the case of accidental release.
3. Is there a local biosafety group, committee, or review board at your institution?
The University of Washington has an Environmental Health and Safety(EHS) committee that deals with biosafety and other safety and health issues. All procedures and materials used are standard, the EHS has no specific concerns. You can visit the EHS at http://www.ehs.washington.edu.
4. Do you have any other ideas how to deal with safety issues that could be useful for future iGEM competitions? How could parts, devices and systems be made even safer through biosafety engineering?
One biosafety measure that would be helpful for many teams would be a standardized bacterial strain with knockout(s) that would require that media be supplemented with a relatively cheap chemical for bacterial growth to occur. This would greatly reduce any risks of accidental release, and virtually eliminate the chances of bacterial growth outside of controlled lab environments. The main difficulty with this approach would be finding a knockout that would not have an impact on growth in controlled laboratory media. This would have an added effect of increasing comparability between projects, as tests could be done in standardized strains.
Responsible for pictures, links and content: Markus Schmidt
Photo Credits (from left to right)
E. coli http://en.wikipedia.org/wiki/File:E-coli-in-color.jpg
 "
"