Team:Washington/Protocols/Display
From 2012.igem.org
Yeast display clonal titration
Contents |
Overview
The principle is to label a clonal population of yeast cells expressing your construct with differing amounts of biotinylated antigen. After an incubation to allow the rxn to reach equilibrium, the cells are washed to remove antigen and labeled with Streptavidin-PE (to monitor binding of antigen) and anti-cymc FITC (to monitor surface expression). Graphs are prepared of the mean PE fluorescence of the surface displayed population of cells as a function of antigen, and the KD is calculated from fits to that data assuming 1:1 binding stoichiometry.
Cell preparation
- Pick a colony of interest and grow overnight in 1 mL SDCAA + CARB/KAN at 30oC shaking overnight
- Spin down cells 2500xg for 3 min and resuspend in 200uL SGCAA + CARB/KAN.
- Spin down the culture again and resuspend in 200uL SGCAA.
- Use 60uL of the resuspension to inoculate 1mL of SGCAA + CARB/KAN.
- Grow overnight at 22oC shaking
- Spin down cells in 2500xg for 3 min, wash with 1 mL PBSF, spin down again, and resuspend in 200uL PBSF. Record OD600 of the sample using nanodrop(remember to multiply by 10) and dilute in PBSF so that OD600=2.0. The concentration of the cells is then 2*104 cells per uL.
Antigen Labeling
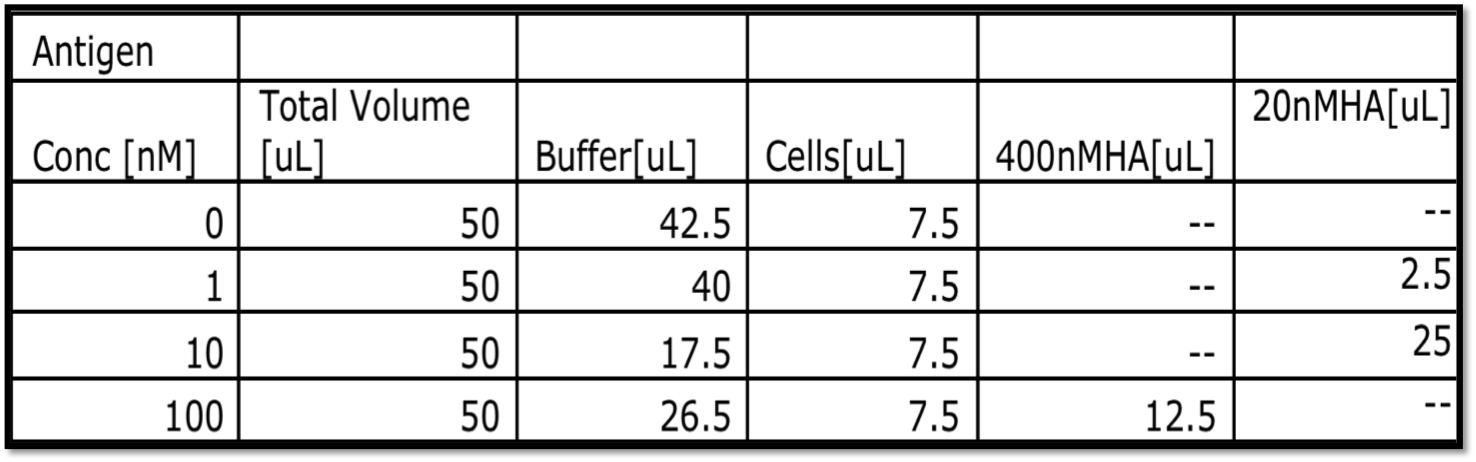
- Label 1.5*105 cells with differing amounts of antigen in a v-bottom 96-well plate. Be sure that the antigen is at least 10x in excess of the number of displayed proteins in the reaction. A sample setup is shown below.
- BEFORE YOU START SETTING UP: Calculate out the total volume of 400nM and 20nM HA you will need for you entire experiment than multiply by 1.1. This is the amount you will need to make starting from the 1uM HA stocks.
- Place a silver cover on the 96-well plate and incubate at 22oC for 30 minutes
EVERYTHING AFTER THIS STEP SHOULD BE DONE WITH COLD REAGENTS!!!
- Spin down plate at 4000rpm for 5 min in plate centrifuge in cold room.
- Make a mastermix containing 0.6uL anticmyc FITC, 0.25uL SAPE and 49.25uL PBSF for each well.
- For 45 wells, this is 27uL FITC, 11.25 uLSAPE, and 2.216 mL PBSF. Make 5-10% more than the number of wells you wish to label.
- Wash each well with 200uL PBSF. Spin down again.
- Pipette 50uL of labeled mastermix to each well. Vortex very briefly and place on ice for 10min.
- Spin down plate at 4000rpm for 5 min in plate centrifuge in cold room.
- Wash each well with 200uL PBSF. Spin down again.
- Leave cells as pellets in 96-well plate on ice.
Reading labeled cells by flow cytometry.
- Using the C6 Flow Cytometer: turn on machine and software.
- Place ddH20 onto sip and set to run for 2min
- Load iGEM2012 template from Aaron folder
- Label sample wells in software to prepare for run
- Resuspend cells in 200uL PBSF into a 1.5mL eppendorf tube.
- Place tube on the sip, lock in place, and hit read.
- Collect 25,000 cells for each sample.
- Be sure to SAVE the data collected!
 "
"