Team:Washington/Flu
From 2012.igem.org
(→Method of computational design) |
|||
| Line 2: | Line 2: | ||
__NOTOC__ | __NOTOC__ | ||
<h1 id='Background'>Background</h1> | <h1 id='Background'>Background</h1> | ||
| + | <center><a href="url">Back To Top</a></center> | ||
The influenza virus, also known as the flu, is a deadly virus that has decimated large populations of various species including humans. However, even with the advent of vaccinations, high rates of mortality still occur because of the constant evolution of the virus. Variations within each strain of influenza are found within the viral surface proteins that coat their surfaces. One such protein is Hemagglutinin (HA), which is represented by the H when describing flu subtypes (H1N1, H2N3, etc). Hemagglutinin is primarily involved in viral transfer as it mediates the binding and entry into a host cell through a sialic acid linkage. Each strain of Influenza is characterized by it's variant of Hemagglutinin. There are 16 known subtypes of HA; these subtypes give rise to the vast multitude of strains that exist to today, as well as future emergent strains. These subtypes usually only differ by a relatively few number of mutations. The World Health Organization uses the history of influenza breakouts to predict upcoming strains which could threaten to cause future pandemics and vaccinations are made using these predictions. | The influenza virus, also known as the flu, is a deadly virus that has decimated large populations of various species including humans. However, even with the advent of vaccinations, high rates of mortality still occur because of the constant evolution of the virus. Variations within each strain of influenza are found within the viral surface proteins that coat their surfaces. One such protein is Hemagglutinin (HA), which is represented by the H when describing flu subtypes (H1N1, H2N3, etc). Hemagglutinin is primarily involved in viral transfer as it mediates the binding and entry into a host cell through a sialic acid linkage. Each strain of Influenza is characterized by it's variant of Hemagglutinin. There are 16 known subtypes of HA; these subtypes give rise to the vast multitude of strains that exist to today, as well as future emergent strains. These subtypes usually only differ by a relatively few number of mutations. The World Health Organization uses the history of influenza breakouts to predict upcoming strains which could threaten to cause future pandemics and vaccinations are made using these predictions. | ||
Revision as of 04:37, 29 September 2012
Background
The influenza virus, also known as the flu, is a deadly virus that has decimated large populations of various species including humans. However, even with the advent of vaccinations, high rates of mortality still occur because of the constant evolution of the virus. Variations within each strain of influenza are found within the viral surface proteins that coat their surfaces. One such protein is Hemagglutinin (HA), which is represented by the H when describing flu subtypes (H1N1, H2N3, etc). Hemagglutinin is primarily involved in viral transfer as it mediates the binding and entry into a host cell through a sialic acid linkage. Each strain of Influenza is characterized by it's variant of Hemagglutinin. There are 16 known subtypes of HA; these subtypes give rise to the vast multitude of strains that exist to today, as well as future emergent strains. These subtypes usually only differ by a relatively few number of mutations. The World Health Organization uses the history of influenza breakouts to predict upcoming strains which could threaten to cause future pandemics and vaccinations are made using these predictions.
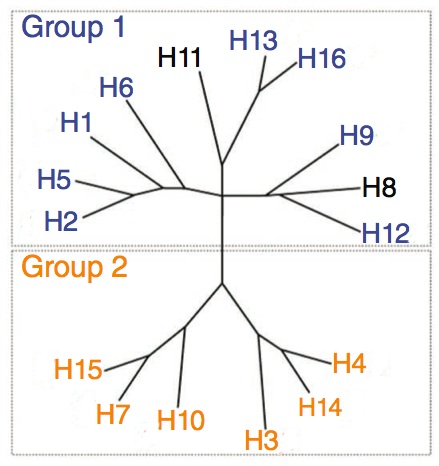
Here are the 16 subtypes of HA. They divided into two groups but we were only working with group 1
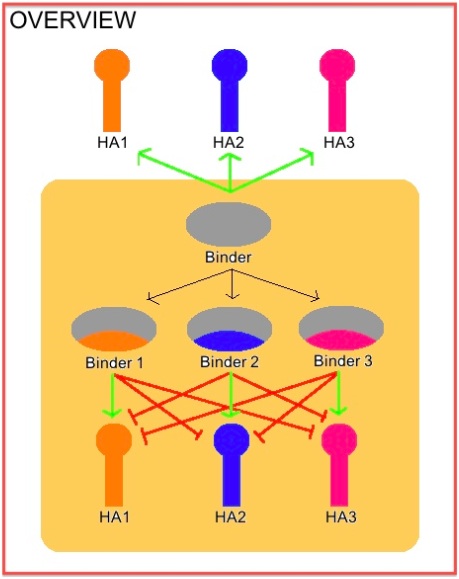
In 2011, Fleishman et al., detailed two small proteins which had binding capabilities to various subtypes of Hemagglutinin (HB80.4 and HB36.5). These protein binders were designed and have been shown to bind to H1. These proteins were later optimized in a paper published in 2012 to increase binding affinity.
Knowing that HB80.4 was already broadly binding to many HAs, we wanted to focus our efforts on the HB36.5 which up until recently had only been tested against H1.
Our goals were to
1) Test HB36.5 binding to subset of common HA proteins
2) Use FoldIt to engineer mutations that would allow variants of HB36.5 to bind preferentially to differnt HA subtypes.
Having two broadly – binding flu binders, with subtype specific capabilities would allow for enhanced rapid and reliable global tracking of current Influenza strains.
Methods
Method of computational design
Gathering information about specific hemagglutinin
Since we would like to improve the binding of our flu binder to specific hemagglutinins, we have to understand the structure of those subtypes. Initially, we searched for specific hemagglutinin structures on Protein Data Bank (http://www.rcsb.org/pdb/home/home.do). If we succeed in finding the desired hemagglutinin structures, we downloaded the PDB file of that hemagglutinin and made a model of the binder bound to it for use in Foldit.
If we could not find the exact structure of a particular hemagglutinin, we would search for the protein sequence of that hemagglutinin on National Center for Biotechnology Information (www.ncbi.nih.gov). We would align the protein sequence of the structurally unknown hemagglutinin we obtained to that of the H1HA(which has a known structure) by using Clustalw2 (http://www.ebi.ac.uk/Tools/msa/clustalw2/). Next, we would make homology models in FoldIt of the desired hemaggluttinin by changing the side chains of the H1HA in Foldit according to the protein sequence differences we found.
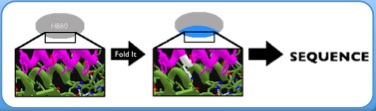
Making model of Foldit
Crystal stuctures of HB36.3 and HB80.4 in complex with H1HA are available on the PDB as PDB ID 3R2X and 4EEF respectively. To create models our the binder bound to different HAs we aligned our alternative HA structures to the H1HA in the published crystal structures using PYMOL. Then, we would record the difference in side chains between that specific hemagglutinin and H1HA and use FoldIt to implement those changes on the known crystal structures. The models we created are linked below:
Proposing mutation on the model
After preparing the Foldit model, we would be able to propose mutations on the flu binder in order to improve its binding to the desired hemagglutinin. There is a score total in Foldit which indicates the energy of the whole protein structure. It is our understanding that the protein structure is more stable when its energy is lower. Thus, our primary goal was to decrease the score total in Foldit. We did that in three different ways, filling the holes, making space, and balancing the electrostatic. Holes that did not exist in H1HA would be created with the replacement of different side chains. We would mutate the corresponding side chain on the flu binder so that it extends out and fill a hole on the hemagglutinin. Making space is the exact opposite of filling the holes. Some side chains of certain hemaggluttinins would be more projected out in space than that of hemagluttinin one. Therefore, we would mutate the corresponding side chains of the flu binder in order to provide room for the more extended side chains. Some variations of side chain on hemaggluttinin causes some electrostatic changes. We would mutate the corresponding side chains of the flu binder so as to counter balance the electrostatic changes. For example, if a certain area on the helix is changed to be more electronegative, we would introduce an electropositive side chain on the flu binder for improving the binding and vice versa.
| File:HB80 H12 model.zip |
|---|
| File:HB80 H9 model.zip |
|---|
| File:HB80 H5 model.zip |
|---|
| File:HB36 H13 model.zip |
|---|
| File:HB36 H12 model.zip |
|---|
| File:HB36 H9 model.zip |
|---|
| File:HB36 H6 model.zip |
|---|
| File:HB36 H5 model.zip |
|---|
| File:HB36 H2 model.zip |
|---|
Testing the mutation
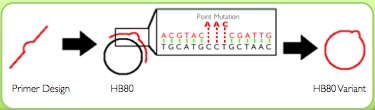
Kunkel’s Mutagenesis
We isolated single stranded DNA (ssDNA) of our vector harboring the wild-type HA gene. To do this we infected cells with bacteriophage M13, which packages its own ssDNA genome identified by length, and so in tandem packaged our vector in single stranded form. We then harvested the phage from the lysed culture of E. coli, and extracted our single stranded vector DNA. Next, we annealed and extended our mutagenic oligos to incorporate the specified mutations into the newly synthesized antisense strand. This hybrid vector was transformed into E. coli that degraded the original uracil-containing DNA and replaced it with sections complementary to the mutagenized strand. (Please see protocol for more details at https://2012.igem.org/Team:Washington/Protocols/Kunkel)
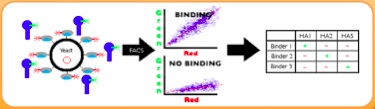
Yeast Transformation
We transformed our mutants into yeast in order to have a more stable cell line for surface display and binding efficiency analysis.
Yeast Surface Display
We first labeled a clonal population of yeast cells expressing the construct with differing amounts of biotinylated antigen. The cells are then washed to remove antigen and labeled with Streptavidin-PE (to monitor binding of antigen) and anti-cymc FITC (to monitor surface expression).
Flow Cytommetry/Fluorescence-Activated Cell Sorting (FACS)
Graphs are prepared of the mean PE fluorescence of the surface displayed population of cells as a function of antigen, and the Kd (dissociation constant) is calculated from fits to that data assuming 1:1 binding stoichiometry. Graphs and KD values are directly proportional to binding efficiency, and doing this experiment at a range of concentrations provides increased accuracy of data.
Results Summary
HB36.5_F317H was predicted to increase HA5 binding but it decreased the binding to HA9. Nevertheless, it can be used for specific hemagglutinin subtypes binder.
HB36.5_H312A was predicted to increase HA2 binding and it did improve binding to HA2.
HB80.4_A280Vwas predicted to decrease HA2 binding but it improved the binding to HA2.
HB80.4_K298E was predicted to increase HA2, HA5, HA6 and HA12 binding and it did improve binding to HA2.
HB80.4_S276H was predicted to increase HA6 binding but it decreased the binding to HA6.
HB80.4_K298S was predicted to increase HA12 binding but it decreased the binding to HA6.
HB80.4_S286K was predicted to increase HA12 binding and it increased the binding to HA12. However, it decreased the binding to HA6.
Future Directions
We plan to combine the HB36.5 mutants H312A and W313A – both successfully having increased binding to HA2 – in order to create a superior combinatorial mutant with multiple-fold improvement. Simultaneously, we will continue the search for mutants that we can use to create combinatorial mutants. These could be used to create a diagnostic test for global health tracking of various strains of influenza, especially those predicted to become epidemic.
Parts Submitted
HB80.4 (Wild-type Flu binder)
HB80.4_S276H
• Predicted to increase HA6 binding.
HB80.4_K298S
• Predicted to increase HA12 binding.
HB80.4_S286K
• Predicted to increase HA12 binding.
HB80.4_K304S
• Predicted to increase HA6 binding.
• Predicted to increase HA12 binding.
• Predicted to increase HA13 binding.
HB80.4_K274R
• Predicted to increase HA13 binding.
HB80.4_A280V
• Predicted to decrease HA2 binding.
HB80.4_K298E
• Predicted to increase HA2 binding.
• Predicted to increase HA5 binding.
• Predicted to increase HA6 binding.
• Predicted to increase HA12 binding.
HB80.4_ K298Q
• Predicted to increase HA13 binding.
HB80.4_Q301K
• Predicted to increase HA2 binding.
HB80.4_Q301R
• Predicted to increase HA2 binding.
• Predicted to increase HA5 binding.
• Predicted to increase HA9 binding.
HB36.5 (Wild-type Flu binder)
HB36.5_A326E
• Predicted to increase HA2 binding.
• Predicted to increase HA5 binding.
• Predicted to increase HA6 binding.
• Predicted to increase HA12 binding.
• Predicted to increase HA13 binding.
HB36.5_A326E + P324K
• Predicted to decrease HA2 binding.
• Predicted to increase HA5 binding.
• Predicted to increase HA6 binding.
• Predicted to increase HA13 binding. HB36.5_F317H
• Predicted to increase HA5 binding.
HB36.5_F317S
• Predicted to increase HA2 binding.
• Predicted to increase HA9 binding.
HB36.5_H312A
• Predicted to increase HA2 binding.
HB36.5_W313A
• Predicted to increase HA2 binding.
 "
"