Team:Wageningen UR/ModifyingtheHepatitisB
From 2012.igem.org
Jjkoehorst (Talk | contribs) (→The VLP) |
Jjkoehorst (Talk | contribs) (→The VLP) |
||
| Line 17: | Line 17: | ||
In this project, we will try to make the attachments of epitopes or ligands even more convenient. The external loop of the Hepatitis B core antigen can be modified, attaching the k-coil to the VLP. Using the PnA System, a wide variety of antigens and ligands can be attached. | In this project, we will try to make the attachments of epitopes or ligands even more convenient. The external loop of the Hepatitis B core antigen can be modified, attaching the k-coil to the VLP. Using the PnA System, a wide variety of antigens and ligands can be attached. | ||
The inside of the VLP can be modified in a similar way, allowing controlled packaging of proteins inside the VLP. | The inside of the VLP can be modified in a similar way, allowing controlled packaging of proteins inside the VLP. | ||
| - | <br><br><br><br><br><br><br><br><br><br><br><br><br> | + | <br><br><br><br><br><br><br><br><br><br><br><br><br><br> |
</p> | </p> | ||
Revision as of 09:45, 26 September 2012
Contents |
Hepatitis B and the Hepatitis B core Antigen VLP
Introduction
The hepatitis B virus is a widespread virus causing liver infection. The prevalence of Hepatitis B is higher in less developed countries, as can be seen in figure 1. About 1% of the population in Western-Europe and Northern America is infected, and up to 5% in less developed countries [1]. The virus can be spread by blood-to-blood contact or as STD (sexually transmitted disease). This is the reason why we do not use full viruses, but the empty shells only. By using the coat proteins only, we prevent transmission.
The VLP
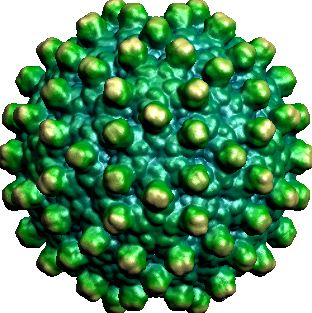
The empty shell, or Virus-Like Particle, is formed by the Hepatitis B core Antigen (HepBcAg), see figure 2. The VLP has already been used for the vaccination against Hepatitis B itself, back in 1980 [3]. Afterwards, the Hepatitis B VLP has been modified and suggested to be used as carrier for epitopes of other pathogens.
In this project, we will try to make the attachments of epitopes or ligands even more convenient. The external loop of the Hepatitis B core antigen can be modified, attaching the k-coil to the VLP. Using the PnA System, a wide variety of antigens and ligands can be attached.
The inside of the VLP can be modified in a similar way, allowing controlled packaging of proteins inside the VLP.
Methods
The template of the coat protein for the Hepatitis B core antigen was supplied by the John Innes centre, in a plasmid usually used for the expression of this protein in plants. We used primers that already included the prefix and suffix of Standard 10, so that after amplification, the gene can be directly ligated in a pSB1C3 backbone. Or, as we did, combined with an inducible promotor and RBS. In order to make the modified VLPs, we designed primers with an overhang containing the modifications. A detailed description of the primers and the modifications can be found in the Journal.
Results
We constructed the BioBrick that containd an inducible promoter, RBS and the gene encoding the wild-type HepBcAg. After expression and purification of the protein, we assembled the VLPs in vivo. The samples were analysed using Electron Microscopy, which yielded the picture below.
References
1. Organization, W.H. Hepatitis B; factsheet No204. 2012 July 2012 [cited 2012 September 23]; Available from: http://www.who.int/mediacentre/factsheets/fs204/en/.
2. E.H., T. Hepatitis B. Infectious Diseases Related To Travel 2011; Available from: http://wwwnc.cdc.gov/travel/yellowbook/2012/chapter-3-infectious-diseases-related-to-travel/hepatitis-b/uganda.htm.
3. Liu, F., et al., Virus-like particles: potential veterinary vaccine immunogens. Res Vet Sci, 2012. 93(2): p. 553-9.
 "
"