Team:Wageningen UR/Journal/week25
From 2012.igem.org
Week 25: 15 october - 21 october
GFP modification
15 October
- 3rd time growing JM109 containing our biobricks BBa_K883702 (GFP coil with an IPTG induced promoter) and BBa_K883703 (GFP coil + His tag with an IPTG induced promoter) and induce expression with IPTG with new medium
-> no GFP production could be seen
16 October
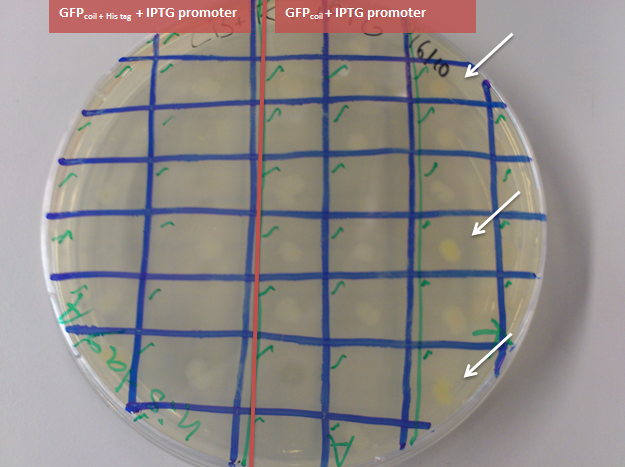
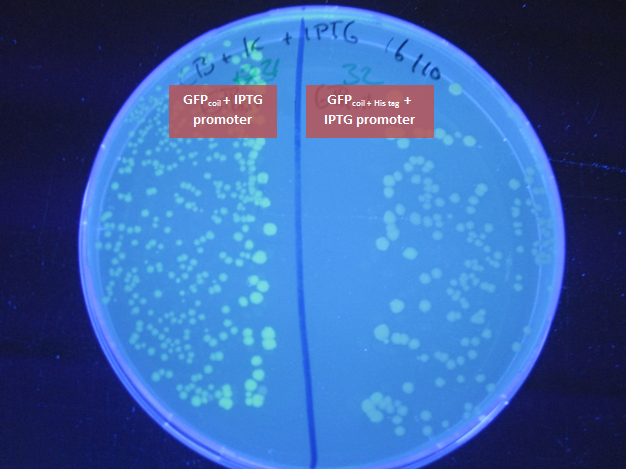
- to check if there are any colonies showing green fluorescence on the original plate (of the transformation on 21.September GFPcoil; GFPcoil + his tag in Bba_J04500 (IPTG induced promoter) in JM109), colonies where picked randomly and plated on agarplates containing IPTG next to the selection antibiotic
-> some colonies containing GFPcoil with an IPTG induced promoter show green fluorescence
- plate the JM109 samples that where used for sending the bricks in to the registry - containing BBa_K883702(GFPcoil with IPTG induced promoter) and BBa_K883703 (GFPcoil + His tag with IPTG induced promoter) on agarplates containing IPTG next to the selection antibiotic
-> culture containing BBa_K883702(GFPcoil with IPTG induced promoter) shows green fluorescence
- grow colonies containing the biobricks BBa_K883702 BBa_K883703 BBa_K883701 and BBa_K883700
- grow colonies containing BBa_K883702 and BBa_K883703 in duplo once with IPTG in the medium and once adding IPTG (fresh stock solution) to the culture at OD=0.6
- miniprep of the colonies containing BBa_K883702 BBa_K883703 BBa_K883701 and BBa_K883700
- 2nd try to send the bricks BBa_K883702 BBa_K883703 BBa_K883701 and BBa_K883700 in for sequencing
-> sequencing revealed that BBa_K883702 and BBa_K883703 had the expected sequence but BBa_K883701 and BBa_K883700 where faulty - therefore we deleted these two bricks from the registry
18 October
- transformation of BBa_K883702 with BL21 (producing strain) - plating the transformants on plates containing IPTG
19 October
- colony PCR of the transformation BBa_K883702 with BL21 using sequencing primers and plating the picked colonies again on a plate containing IPTG
-> all the colonies picked had the correct insert and showed fluorescence under the UV light
- transformation of BBa_K883703 (GFPcoil + his tag + IPTG promoter) with BL21 (producing strain)
- digestion of BBa_K883702 as well as BBa_K883703 and ligation with the pSB1C3 backbone (originating from BBa_J04450). These constructs where than transformed with DH5α and plated on plate containing IPTG.
- plate JM109 containing the faulty brick BBa_K883700 and BBa_K883701 again to check if there are different colonies present containing different inserts
-> on the plate containg BBa_K883700 there where even red colonies growing on the plate (from original plasmid BBa_J04450 of the pSB1C3 backbone and encode for RFP with a constitutive promoter) so clearly there was a mistake done with picking single colonies from the tranformants
21 October
- innoculate 2x45ml medium with 10ml culture containing GFPcoil with an IPTG promoter. After an OD of around 0.5 was reached IPTG was added and the batch was incubated overnight
-> no fluorescence could be seen in the fluid or the pellet
- Colony PCR of the transformation from 19. October (BBa_K883703 (GFPcoil + his tag + IPTG promoter) with BL21; BBa_K883702 and BBa_K883703 in the pSB1C3 backbone

Figure 6: transformation of the ligation of BBa_K883702 with the pSB1C3 backbone in DH5α. The red colonies contain the original plasmid BBa_J04450 of the pSB1C3 backbone and encode for RFP with a constitutive promoter, the green colonies contain the successful ligation product with the RFP encoding gene cut out and the GFPcoil + IPTG promoter ligated into the backbone (examples marked with an arrow). The circled ones are picked colonies'
-> comapring these two outcomes together it can be concluded that colony nr. 1 of the GFPcoil in pSB1C3 backbone in DH5α transformants (first lane second row in figure 5; left arrow to the circle in picture 6) has the correct insert and the GFP is functioning
- pick white colonies from the plate with JM109 containing the faulty brick BBa_K883700 and BBa_K883701 (from 19.october) and plate them again to seperate the cultures
Hepatitis B inside modification
15 October
- PCR check of the PCR fragment obtained on 27.August
- ligation of the PCR product in pJET
- transformation with DH5α
-> there was growth on the negative control plate
CCMV K-coil frameshift correction
Directly after the European Jamboree we ordered two new primers to correct the frameshifts of the CCMV wt K-coil construct and the CCMV Δ26 K-coil construct. With these two primers we are able to repeat our extension-PCR scheme. To prevent new and unwanted frameshifts the primers were designed to anneal at the BBa_K883000 which is coding for the wildtype coat protein of CCMV and has a confirmed DNA-sequence. Additionally the resulting constructs were checked with Expasy translate to find unnecessary STOP-codons.
CCMV wt K-coil FW1: 5' TGGTGGTGGTTCTGGTGGTGGTGGTTCTGCTGCTGCTATGGGTACAGTCGGAACAGG 3'
CCMV Δ26 K-coil FW1: 5'TGGTGGTGGTTCTGGTGGTGGTGGTTCTGCTGCTGCTGTGGTCCAACCTGTTATTGTAGA 3'
PCR step I PCR step II PCR step III PCR step IV
 "
"