Team:Wageningen UR/Journal/week12
From 2012.igem.org
(Difference between revisions)
MarkvanVeen (Talk | contribs) |
(→week 12: 16 july - 22 july) |
||
| Line 14: | Line 14: | ||
== Lab work == | == Lab work == | ||
| - | + | '''General''' | |
| + | |||
| + | BBa_J04450 (RFP coding device) transformation, digestion check | ||
| + | |||
| + | *BBa_J04450 was solubilized from the Standard registry parts plate I with 10µl MQ water | ||
| + | *transformation of electrocompetent ''E.coli'' with the plasmid | ||
| + | *plated on LB-agar with corresponding antibiotic (chloramphenicol) | ||
| + | *6 red-fluorescing colony were picked after overnight growth in 37°C | ||
| + | *subsequent miniprep | ||
| + | |||
| + | {| class="wikitable" style="text-align: center" | ||
| + | |- style="font-style: italic" | ||
| + | |sample | ||
| + | |concentration (ng/µl) | ||
| + | |260/280 | ||
| + | |- | ||
| + | |A1 | ||
| + | |115.5 | ||
| + | |1.92 | ||
| + | |- | ||
| + | |C1 | ||
| + | |96.0 | ||
| + | |1.98 | ||
| + | |- | ||
| + | |C3 | ||
| + | |100.1 | ||
| + | |1.92 | ||
| + | |} | ||
| + | |||
| + | digestion check with EcoRI and SpeI | ||
| + | |||
''' HepB ''' | ''' HepB ''' | ||
Revision as of 12:43, 24 September 2012
week 12: 16 july - 22 july
Office work
PLRV
This week we designed and ordered all the primers we needed.
Lab work
General
BBa_J04450 (RFP coding device) transformation, digestion check
- BBa_J04450 was solubilized from the Standard registry parts plate I with 10µl MQ water
- transformation of electrocompetent E.coli with the plasmid
- plated on LB-agar with corresponding antibiotic (chloramphenicol)
- 6 red-fluorescing colony were picked after overnight growth in 37°C
- subsequent miniprep
| sample | concentration (ng/µl) | 260/280 |
| A1 | 115.5 | 1.92 |
| C1 | 96.0 | 1.98 |
| C3 | 100.1 | 1.92 |
digestion check with EcoRI and SpeI
HepB
- digestion of the HepB core protein PCR product with pre- and suffix (from 11.July) and ligation into BBa_J04500 (an IPTG inducible promoter with RBS)as well as BBa_PSB1K3.ml (linearized plasmid backbone)
- electro transformation of these constructs with DH5α
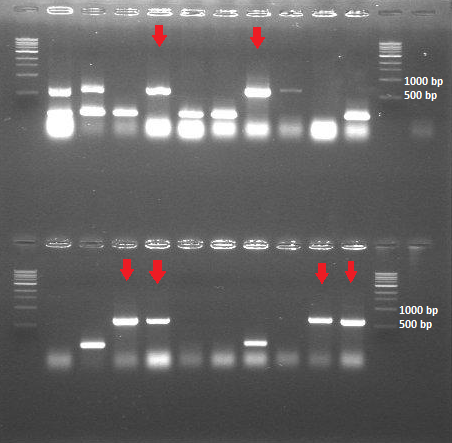
- colony PCR (20 samples of the transformants containing HepB core protein + BBa_J04500; 10 samples of the transformants containing HepB core protein + BBa_PSB1K3.ml
-> the ligation and transformation of the HepB core protein into the linear backbone (BBa_PSB1K3.ml) was not successful -> the colony PCR shows that we have 6 out of 20 colonies with the expected insert size (around 600bp)
TuYV
- Mach1 electrocompetent E. coli cells were transformed with last week's ligation mixtures (TuYV coat protein and TuYV coat protein with his-tag). Results were unsatisfying. No colonies were showed on the sample plates.
- 'Coat Protein + readthrough' gene amplicons were digested, ligated into pSB1K3 backbone and transformed into our electrocompetent Mach1 E. coli cells. After two attempts and a colony PCR, results were still unsatisfying.
PLRV
After a two week quest for PLRV infected plant material, we obtained infected potato leafs from the Dutch General Inspection Service for Agricultural seed and seed potatoes.
DLS boundary experiment
19th July (Mark)
- DLS measurment: 20 runs, 45 seconds
- Changed 4 times to different vials to see if the vials are important to the measurment
 "
"