Team:Wageningen UR/Journal/week10
From 2012.igem.org
(Difference between revisions)
MarkvanVeen (Talk | contribs) |
Hanyue0731 (Talk | contribs) (→Office work) |
||
| (7 intermediate revisions not shown) | |||
| Line 2: | Line 2: | ||
= week 10: 2 july - 8 july = | = week 10: 2 july - 8 july = | ||
| - | |||
| - | |||
| - | |||
| Line 12: | Line 9: | ||
Monday: | Monday: | ||
| - | * A new transformation of last | + | * A new transformation of last week's ligation mixes into our electro-competent DH5 Alfa ''E. Coli'' was performed. The next day we checked the colony, there were many colonies on the positive control, however there were the same level of colonies on the sample and negative control. The transformation was contaminated. |
* Because there are iGEM illegal sites in our TuYV readthrough part, we designed a new construct named 4 and 4H, which were coat protein with part of the readthrough without illegal site and coat protein with part of the readthrough without illegal site with his-tag. | * Because there are iGEM illegal sites in our TuYV readthrough part, we designed a new construct named 4 and 4H, which were coat protein with part of the readthrough without illegal site and coat protein with part of the readthrough without illegal site with his-tag. | ||
| Line 21: | Line 18: | ||
* Today we successfully PCR amplified the construct 4, which should be around 800bp. | * Today we successfully PCR amplified the construct 4, which should be around 800bp. | ||
| - | [[File:PCR amplification of 4.jpg|frame|center|Figure 1: | + | [[File:PCR amplification of 4.jpg|frame|center|Figure 1: Isolation of TuYV construct 4 from the TuYV genome]] |
Wednesday: | Wednesday: | ||
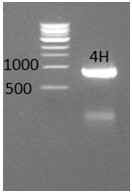
* Today we tried another time to PCR amplify the construct 4H and we got expected band on the gel check. | * Today we tried another time to PCR amplify the construct 4H and we got expected band on the gel check. | ||
| - | [[File:PCR amplification of 4H.jpg|frame|center|Figure 2: | + | [[File:PCR amplification of 4H.jpg|frame|center|Figure 2: Isolation of TuYV construct 4H from the TuYV genome]] |
| Line 49: | Line 46: | ||
---- | ---- | ||
| + | |||
| + | '''FPLC S400 preparations''' | ||
| + | |||
| + | |||
| + | ''2nd – 5th July'' (Mark) | ||
| + | <ul> | ||
| + | <li>Pouring and running of the S400 FPLC with the help of Serve Kengen.</li> | ||
| + | </ul> | ||
| + | |||
| + | |||
| + | ''6th July'' (Mark) | ||
| + | <ul> | ||
| + | <li>FPLC with the S400 column</li> | ||
| + | <li>1 run with 0.5 ml sample</li> | ||
| + | <li>A very good separation with low concentration</li> | ||
| + | </ul> | ||
| + | |||
| + | ---- | ||
| + | |||
[[https://2012.igem.org/Team:Wageningen_UR/Journal/week9 previous week]] [[https://2012.igem.org/Team:Wageningen_UR/Journal/week11 next week]] | [[https://2012.igem.org/Team:Wageningen_UR/Journal/week9 previous week]] [[https://2012.igem.org/Team:Wageningen_UR/Journal/week11 next week]] | ||
Latest revision as of 22:59, 26 September 2012
week 10: 2 july - 8 july
Lab work
TuYV
Monday:
- A new transformation of last week's ligation mixes into our electro-competent DH5 Alfa E. Coli was performed. The next day we checked the colony, there were many colonies on the positive control, however there were the same level of colonies on the sample and negative control. The transformation was contaminated.
- Because there are iGEM illegal sites in our TuYV readthrough part, we designed a new construct named 4 and 4H, which were coat protein with part of the readthrough without illegal site and coat protein with part of the readthrough without illegal site with his-tag.
- We tried to isolate and amplify 4 and 4H today, but we did not get any band on the agrose gel check
Tuesday:
- Today we successfully PCR amplified the construct 4, which should be around 800bp.
Wednesday:
- Today we tried another time to PCR amplify the construct 4H and we got expected band on the gel check.
DLS boundary experiment
6th July (Mark)
- Growing CCMV for the temperature and pH stability experiment
- Inoculated 200 mL of LB with BL21 that contains the IPTG inducible CCMV wild type monomer
- Followed protocol of producing CCMV
7th – 10th July (Mark)
- Dialysis CCMV VLPs
- Now running 1 times 50 mL dialysis tubes with CCMV wild type
- Followed protocol of dialysis of CCMV
FPLC S400 preparations
2nd – 5th July (Mark)
- Pouring and running of the S400 FPLC with the help of Serve Kengen.
6th July (Mark)
- FPLC with the S400 column
- 1 run with 0.5 ml sample
- A very good separation with low concentration
 "
"