Team:Wageningen UR/Journal/insilico
From 2012.igem.org
In silico formation of subunits
While waiting for the Hepatitis B core antigen gene and the sequence, we prepared the possible modifications and tried to visualise the outcome of these modifications. Therefore, the first weeks of the Hepatitis B lab journal mainly contains in silico work.
May 9
Tertiary structure modelling for the prediction of modification in subunits
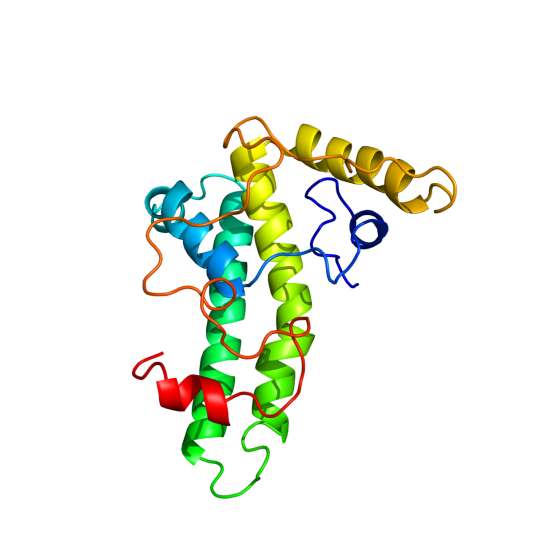
To determine what the final tertiary structure of the subunits could look like, we use Phyre2 (http://www.sbg.bio.ic.ac.uk/phyre2/html/page.cgi?id=index). First of all, the wild type Hepatitis B Core Antigen (HBcAg) was analysed by the server. Amino acid sequence used was Hepatitis B core antigen [synthetic construct] (VERSION ABI31779.1 GI:113197025). The result had a large region with high certainty prediction: 77% with over 90% certainty
Hepatis B core antigen synthetic construct
Synthetic aa sequence mdidpykefgatvellsflpsdffpsvrdlldtasalyrealespehcsphhtalrqailcwgelmtlatwvgnnledp((insertlocation))asrdlvvnyvntnvglkirqllwfhiscltfgretvleylvsfgvwirtppayrppnapilstlpettvvrrrdrgrsprrrtpsprrrrspsprrrrsqsresqc
Hepatitis B has previously been modified with the 5EPIS antigen (ref). The amino acid sequence from this modification was also analysed by Phyre2, knowing it still forms a VLP. This should give an insight in the boundaries of subunit modification.
Found the Viper Database for Virus like particles (http://viperdb.scripps.edu/). This database gives a visualisation of the structure of Virus-Like Particles, but only contains CCMV and HepBcAg, not PoLeRo.
May 15
Modifications
Inserted the E-coil, K-coil and MRSA antigen in the loop and used Phyre2 to predict the tertiary structure. None of the inserts changed the wild type structure of the protein, but the MRSA antigen wrapped around the external loop, possibly inhibiting VLP formation. Both coils appear to be bended, while they are designed to be used linear.
May 16
Inserted the HIV GP120 antigen in de loop. Result was the same as with the MRSA antigen yesterday.
May 18
We tried to use the Pisa PDBe server (http://www.ebi.ac.uk/msd-srv/prot_int/pistart.html) to predict the quaternary structure of the proteins, which is in our case the full VLP. We used the prediction from the synthetic construct. We did not manage to retrieve it, probably because of missing information in the .pdb file given as output from the Phyre2 assignment.
May 22
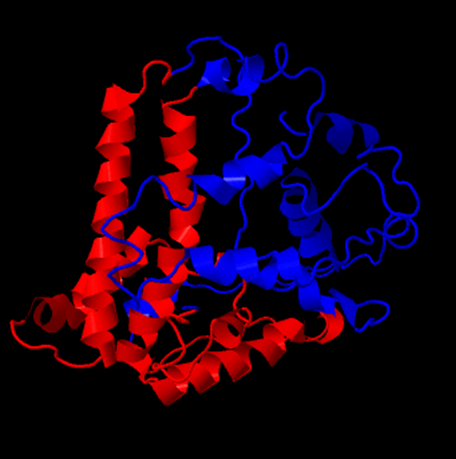
Chimera 1.6.1 is used to assemble subunits of VLPs to full VLPs. When the full .pdb file for the whole VLP is used, the VLP is formed in silico. But, it takes a while on the provided PC and analysis is difficult because of the needed computational power. Even turning around the VLP is only possible at around one frame per minute.
May 30
Send an e-mail to the PDBe contact (Matthew Conroy) about the use of PISA. He advised to use Chimera.
June 2
By adding the crystal structure from the Viper Database pdb file (1qgt), it was possible to turn a unmodified 4 chain Hepatitis B core antigen into a full VLP. This was done by selecting the “multiscale models” panel and changing the threshold atom density to 0.002. After choosing the Biological Unit the model was recreated that is also shown on the viper database.
June 5
Used linkers to make the coils linear. In general, the longer the spacers the more linear the coil becomes. However, when using a combination of a long flexible linker (FL4) and a rigid helical linker (HL2), the construct becomes that big that the coil is wrapped around the protein, probably inhibiting VLP formation. So, a balance needs to be found between length of the linker and linearity of the coil. The sequences used can be found below. In green the amino acid sequence for the k-coil, in capitals on either site the sequence of the different flexible linkers (FL), short linker (SL) and helical linkers (HL). The number indicates the length of the linker, the number of repeats. All these amino acid sequences were analysed using Phyre2.
SL: mdidpykefgatvellsflpsdffpsvrdlldtasalyrealespehcsphhtalrqailcwgelmtlatwvgnnledpLAAAKIAALKEKIAALKEKIAALKELAAAasrdlvvnyv ntnvglkirqllwfhiscltfgretvleylvsfgvwirtppayrppnapilstlpettvvrrrdrgrsprrrtpsprrrrspsprrrrsqsresqc
In orange circle the bended k-coil
FL3: mdidpykefgatvellsflpsdffpsvrdlldtasalyrealespehcsphhtalrqailcwgelmtlatwvgnnledpLGGGGSGGGGSGGGGSAAAKIAALKEKIAALKEKIAALK ELGGGGSGGGGSGGGGSAAAasrdlvvnyvntnvglkirqllwfhiscltfgretvleylvsfgvwirtppayrppnapilstlpettvvrrrdrgrsprrrtpsprrrrspsprrrrsqsresqc
The coil (in orange circle) is still bended, but less compared to the SL construct
FL4: mdidpykefgatvellsflpsdffpsvrdlldtasalyrealespehcsphhtalrqailcwgelmtlatwvgnnledpLSGGGGSGGGGSGGGGSGGGGSAAAKIAALKEKIAALKE KIAALKELSGGGGSGGGGSGGGGSGGGGSAAA asrdlvvnyvntnvglkirqllwfhiscltfgretvleylvsfgvwirtppayrppnapilstlpettvvrrrdrgrsprrrtpsprrrrspsprrrrsqsresqc
The coil is straight, but wrapped around the subunit
HL2: mdidpykefgatvellsflpsdffpsvrdlldtasalyrealespehcsphhtalrqailcwgelmtlatwvgnnledpLAEAAAKEAAAKAAAKIAALKEKIAALKEKIAALKELAE AAAKEAAAKAAAasrdlvvnyvntnvglkirqllwfhiscltfgretvleylvsfgvwirtppayrppnapilstlpettvvrrrdrgrsprrrtpsprrrrspsprrrrsqsresqc
The helical coil pushes the coil to the top of the subunit, which corresponds with the outside of the VLP
HL3: mdidpykefgatvellsflpsdffpsvrdlldtasalyrealespehcsphhtalrqailcwgelmtlatwvgnnledpLAEAAAKEAAAKEAAAKAAAKIAALKEKIAALKEKIAAL KELAEAAAKEAAAKEAAAKAAAasrdlvvnyvntnvglkirqllwfhiscltfgretvleylvsfgvwirtppayrppnapilstlpettvvrrrdrgrsprrrtpsprrrrspsprr rrsqsresqc
The coil is straight but probably inhibiting VLP formation. With HL4 anf HL5 this problem becomes bigger.
HL4: mdidpykefgatvellsflpsdffpsvrdlldtasalyrealespehcsphhtalrqailcwgelmtlatwvgnnledpLAEAAAAAKEAAAKEAKEAAAKAAAKIAALKEKIAALKE KIAALKELAEAAAKEAAAKEAAAKEAAAKAAAasrdlvvnyvntnvglkirqllwfhiscltfgretvleylvsfgvwirtppayrppnapilstlpettvvrrrdrgrsprrrtpsp rrrrspsprrrrsqsresqc
HL5: mdidpykefgatvellsflpsdffpsvrdlldtasalyrealespehcsphhtalrqailcwgelmtlatwvgnnledpLAEAAAKEAAAKEAAAKEAAAKEAAAKAAAKIAALKEKI AALKEKIAALKELAEAAAKEAAAKEAAAKEAAAKEAAAKAAAasrdlvvnyvntnvglkirqllwfhiscltfgretvleylvsfgvwirtppayrppnapilstlpettvvrrrdrg rsprrrtpsprrrrspsprrrrsqsresqc So, a balance needs to be found between length of the linker and linearity of the coil, preferably with the help of a short helical linker (HL2) to push the coil to the outside of the VLP.
HL2/FL2/E.coil/FL2/HL2
mdidpykefgatvellsflpsdffpsvrdlldtasalyrealespehcsphhtalrqailcwgelmtlatwvgnnledpLAEAAAKEAAAKAAALGGGGSGGGGSAAAKIAALKEKIA ALKEKIAALKELGGGGSGGGGSAAALAEAAAKEAAAKAAAasrdlvvnyvntnvglkirqllwfhiscltfgretvleylvsfgvwirtppayrppnapilstlpettvvrrrdrgrs prrrtpsprrrrspsprrrrsqsresqc
Conclusions
The linkers do not have the wanted effect, the coil is not on the outside and VLP formation could be inhibited. Unfortunately, this construct is too big and does not expose the coil on the outside of the VLP. Therefor we will try (A) an intermediate flexible linker to give the coil the flexibility to move to the oppositely charged coil, and (B) the insertion of a Tobacco Ets Virus (TEV) protease site on one site and a short linker on the other site.
 "
"