Team:University College London/Research/MarineBacteria
From 2012.igem.org
(→References) |
(→Growth comparison) |
||
| (16 intermediate revisions not shown) | |||
| Line 3: | Line 3: | ||
= Marine Chassis = | = Marine Chassis = | ||
| - | ==Roseobacter clade bacteria | + | ==Roseobacter clade bacteria == |
| - | |||
| - | + | In consultation with Paola R. Gomez-Pereira of the National Oceanography Centre, Southampton, we identified ''Oceanibulbus indolifex'' and ''Roseobacter dentitrificans'' as promising chassis for the expression of our systems. | |
| - | + | The roseobacter clade bacteria, RCB, constitute a significant proportion of coastal and ocean bacterioplankton communities, estimated above 20% and 15% respectively, and are found in a diverse range of marine habitats. (Buchan ''et al.'' 2006). RCB demonstrate numerous traits, including aerobic anoxygenic phototrophy, aromatic compound degradation and recycling of sulphur within the water column and have been implicated in carbon monoxide consumption. (Wagner-Döbler & Biebl, ''et al.'' 2006). Significantly, in the context of plastic Island, they are shown to be major colonisers of submerged surfaces in marine waters (Dang & Lovell ''et al.'' 2002). | |
| - | |||
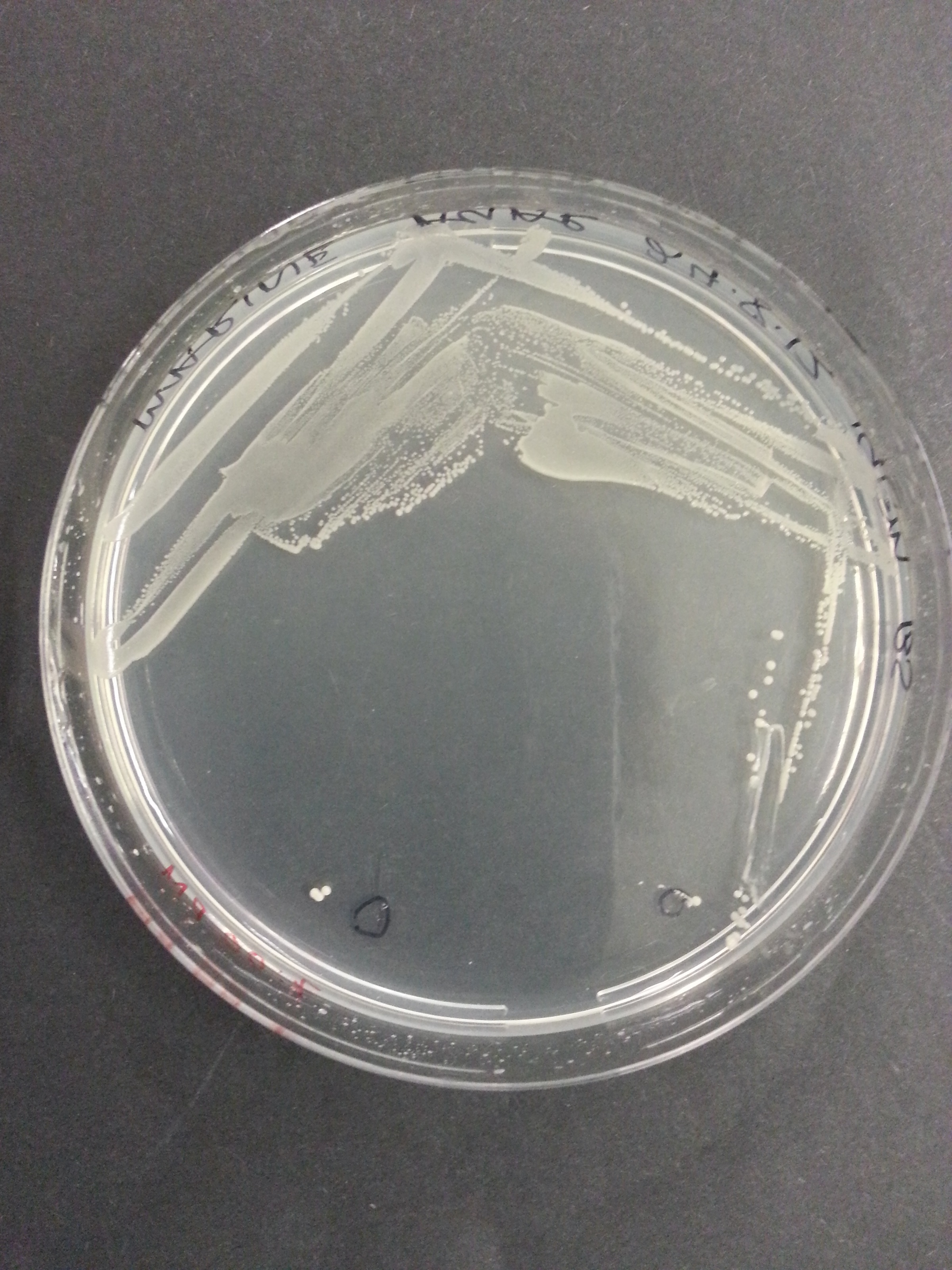
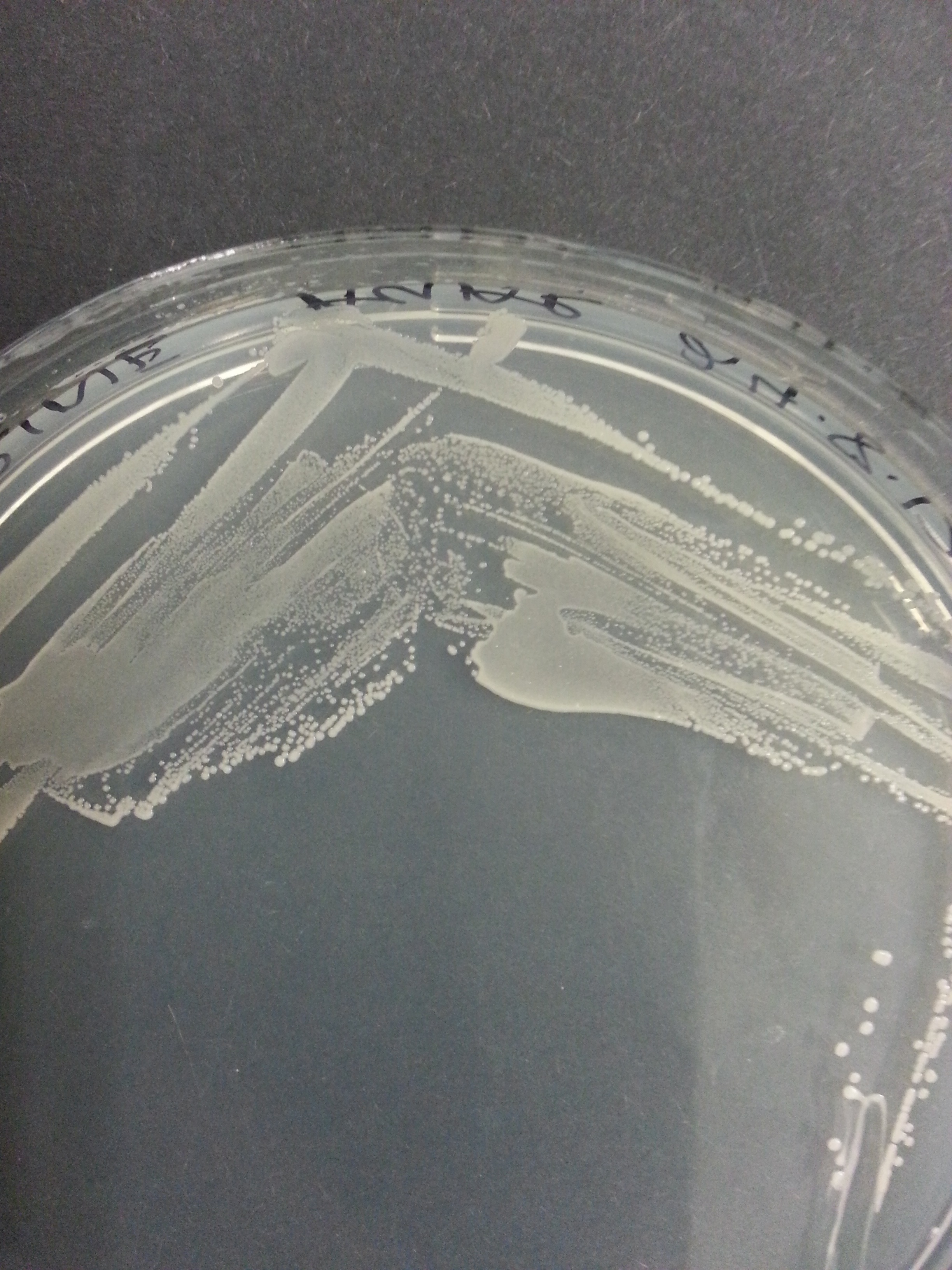
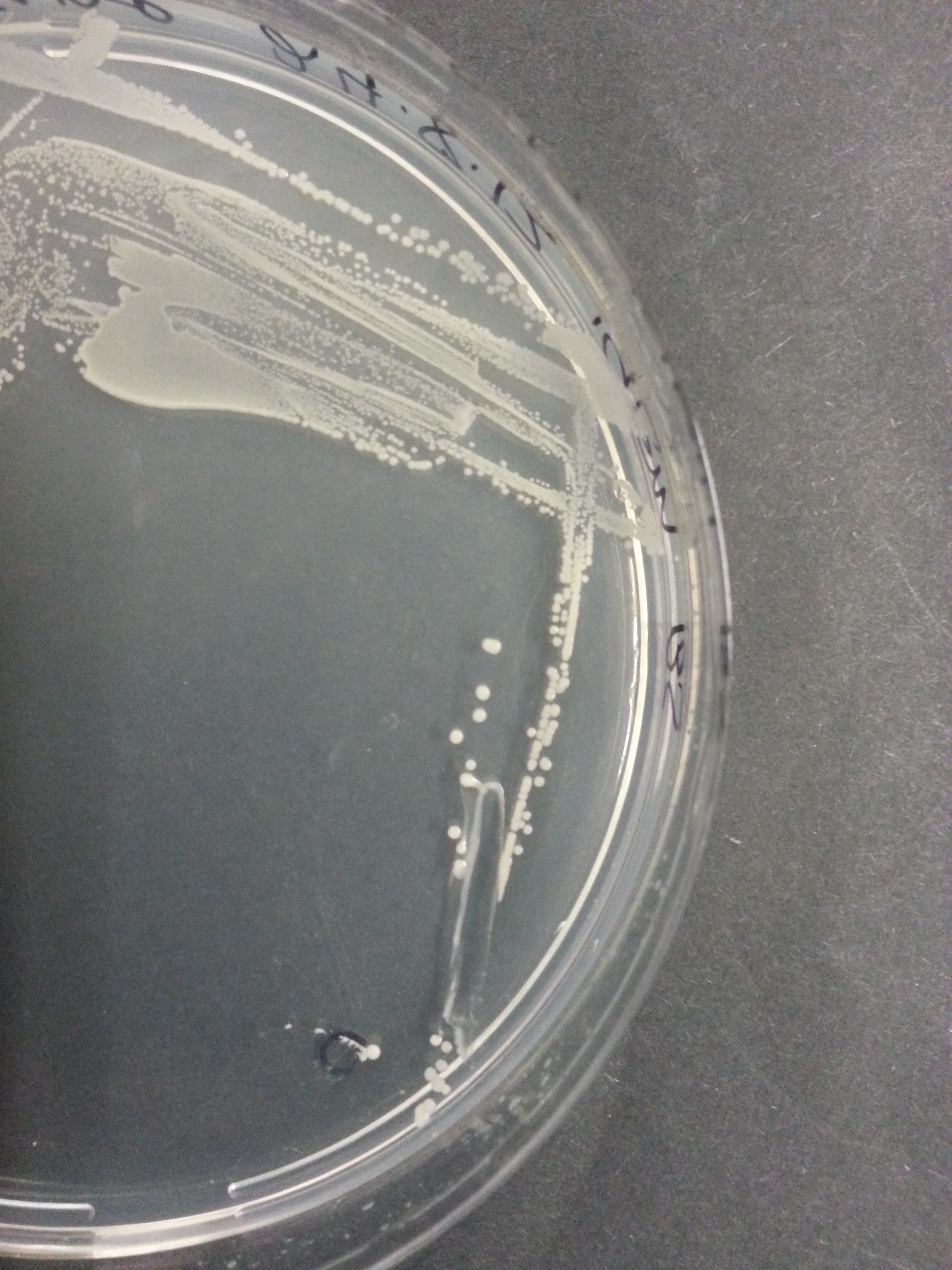
[[File:UniversityCollegeLondon_O_indolifex_Transformed_Wide.jpg|310px]] [[File:UniversityCollegeLondon_O_indolifex_Transformed_Medium.jpg|310px]] [[File:UniversityCollegeLondon_O_indolifex_Transformed_Close.jpg|310px]] | [[File:UniversityCollegeLondon_O_indolifex_Transformed_Wide.jpg|310px]] [[File:UniversityCollegeLondon_O_indolifex_Transformed_Medium.jpg|310px]] [[File:UniversityCollegeLondon_O_indolifex_Transformed_Close.jpg|310px]] | ||
| + | |||
| + | Figure 1. ''Oceanibulbus indolifex''streaked on marine agar, showing colony morphology. | ||
==Transformation Protocols== | ==Transformation Protocols== | ||
| - | Transformation of ''Oceanibulbus indoliflex'' by electroporation. | + | Transformation of ''Oceanibulbus indoliflex'' by electroporation. Adapted from (Piekaski ''et al.'' 2009) |
Electrocompetent cells were prepared according to the following protocol: | Electrocompetent cells were prepared according to the following protocol: | ||
| - | + | 45ml Marine Broth was inoculated with 1ml of ''O. indoliflex'' and grown to ~ 0.5 at OD 578. | |
| - | + | 10ml volumes were transferred to 50 mL falcons and cells were sedimented for 15mins at 3200 x g in a pre-chilled centrifuge. | |
Cells were washed 5 times with 10% (v/v) glycerol, and finally resuspended in 400uL 10% (v/v) glycerol. | Cells were washed 5 times with 10% (v/v) glycerol, and finally resuspended in 400uL 10% (v/v) glycerol. | ||
50uL aliquots were made and stored at -80C. | 50uL aliquots were made and stored at -80C. | ||
| + | |||
| + | Electroporation: | ||
1uL DNA was added to 50uL competent cells to chilled 1mm electrocuvettes, and treated with a field strength of 2.5kV. | 1uL DNA was added to 50uL competent cells to chilled 1mm electrocuvettes, and treated with a field strength of 2.5kV. | ||
| Line 36: | Line 38: | ||
==Growth comparison== | ==Growth comparison== | ||
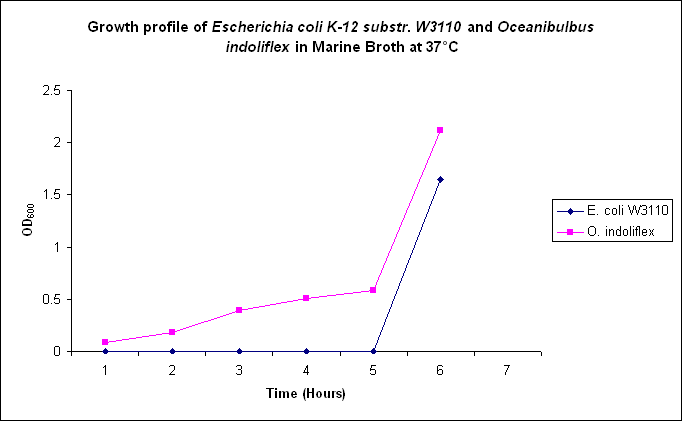
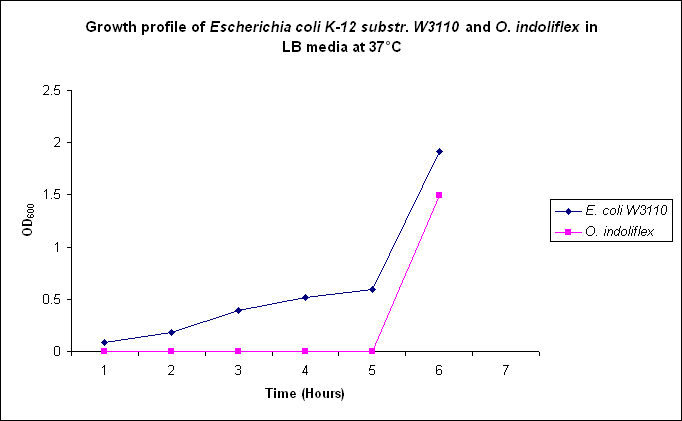
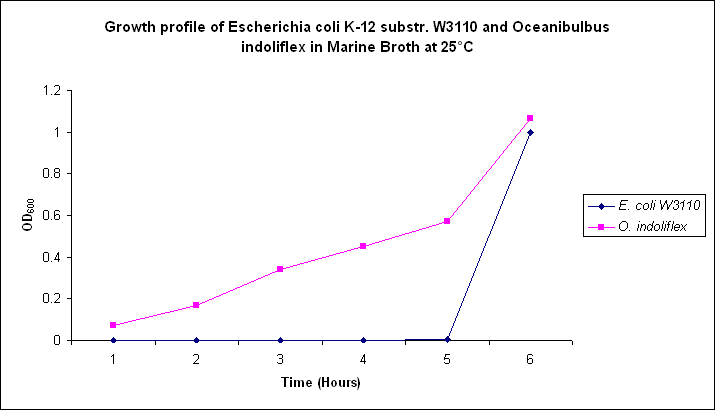
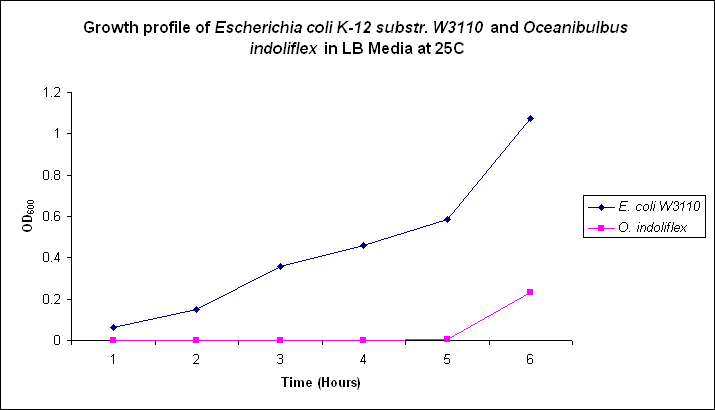
| - | Growth comparison of ''Oceanibulbus | + | Growth comparison of ''Oceanibulbus indolifex'' and ''Escherichia coli'' in marine and luria broth. |
| - | We sought to compare the growth profiles of ''O. | + | We sought to compare the growth profiles of ''O. indolifex'' and ''E. coli''. |
[[File:UniversityCollegeLondon_Marine_1.png|478px]] | [[File:UniversityCollegeLondon_Marine_1.png|478px]] | ||
| Line 43: | Line 45: | ||
[[File:UniversityCollegeLondon_Marine_3.png|478px]] | [[File:UniversityCollegeLondon_Marine_3.png|478px]] | ||
[[File:UniversityCollegeLondon_Marine_4.png|478px]] | [[File:UniversityCollegeLondon_Marine_4.png|478px]] | ||
| + | |||
| + | Figures 2-5. Comparative growth profiles of ''Escherichia coli K-12 substr. W3110'' and ''Oceanibulbus indolifex'' in Marine Broth and LB media at 25°C and 37°C. | ||
==References== | ==References== | ||
Latest revision as of 14:21, 15 October 2012
Contents |
Marine Chassis
Roseobacter clade bacteria
In consultation with Paola R. Gomez-Pereira of the National Oceanography Centre, Southampton, we identified Oceanibulbus indolifex and Roseobacter dentitrificans as promising chassis for the expression of our systems.
The roseobacter clade bacteria, RCB, constitute a significant proportion of coastal and ocean bacterioplankton communities, estimated above 20% and 15% respectively, and are found in a diverse range of marine habitats. (Buchan et al. 2006). RCB demonstrate numerous traits, including aerobic anoxygenic phototrophy, aromatic compound degradation and recycling of sulphur within the water column and have been implicated in carbon monoxide consumption. (Wagner-Döbler & Biebl, et al. 2006). Significantly, in the context of plastic Island, they are shown to be major colonisers of submerged surfaces in marine waters (Dang & Lovell et al. 2002).
Figure 1. Oceanibulbus indolifexstreaked on marine agar, showing colony morphology.
Transformation Protocols
Transformation of Oceanibulbus indoliflex by electroporation. Adapted from (Piekaski et al. 2009)
Electrocompetent cells were prepared according to the following protocol:
45ml Marine Broth was inoculated with 1ml of O. indoliflex and grown to ~ 0.5 at OD 578.
10ml volumes were transferred to 50 mL falcons and cells were sedimented for 15mins at 3200 x g in a pre-chilled centrifuge.
Cells were washed 5 times with 10% (v/v) glycerol, and finally resuspended in 400uL 10% (v/v) glycerol.
50uL aliquots were made and stored at -80C.
Electroporation:
1uL DNA was added to 50uL competent cells to chilled 1mm electrocuvettes, and treated with a field strength of 2.5kV.
1ml marine broth was added to cells and mixtures were transferred to falcons for incubation overnight at room temperature at 200rpm and finally plated on marine agar supplemented with appropriate antibiotics. Incubated at 30˚C for 2 days.
Growth comparison
Growth comparison of Oceanibulbus indolifex and Escherichia coli in marine and luria broth. We sought to compare the growth profiles of O. indolifex and E. coli.
Figures 2-5. Comparative growth profiles of Escherichia coli K-12 substr. W3110 and Oceanibulbus indolifex in Marine Broth and LB media at 25°C and 37°C.
References
1. Brinkhoff, T., Giebel, H.-A., & Simon, M. (2008). Diversity, ecology, and genomics of the Roseobacter clade: a short overview. Archives of microbiology, 189(6), 531–9.
2. Piekarski, T., Buchholz, I., Drepper, T., Schobert, M., Wagner-Doebler, I., Tielen, P., & Jahn, D. (2009). Genetic tools for the investigation of Roseobacter clade bacteria. BMC microbiology, 9, 265.
3. Buchan, A., González, J. M., & Moran, M. A. (2005). Overview of the marine roseobacter lineage. Applied and environmental microbiology, 71(10), 5665–77.
4. Dang, H., & Lovell, C. R. (2002). Seasonal dynamics of particle-associated and free-living marine Proteobacteria in a salt marsh tidal creek as determined using fluorescence in situ hybridization. Environmental microbiology, 4(5), 287–95.
5. Wagner-Döbler, I., & Biebl, H. (2006). Environmental biology of the marine Roseobacter lineage. Annual review of microbiology, 60, 255–80.
 "
"