Team:University College London/Module 6/Results
From 2012.igem.org
Erinoerton (Talk | contribs) (→Colony Halo size Assay) |
Erinoerton (Talk | contribs) (→Colony Halo size Assay) |
||
| Line 29: | Line 29: | ||
{| class="bigtable" | {| class="bigtable" | ||
|- | |- | ||
| - | ! Time !! Sample 1(Abs) ! Sample 2 (Abs) | + | ! Time !! Sample 1(Abs) !! Sample 2 (Abs) |
|- | |- | ||
| 0 || 0.001 || 0.001 | | 0 || 0.001 || 0.001 | ||
| Line 61: | Line 61: | ||
{| class="bigtable" | {| class="bigtable" | ||
|- | |- | ||
| - | ! Time !! Sample 1(Abs) ! Sample 2 (Abs) | + | ! Time !! Sample 1(Abs) !! Sample 2 (Abs) |
|- | |- | ||
| 0 || 0.001 || 0.001 | | 0 || 0.001 || 0.001 | ||
Revision as of 19:36, 26 September 2012
Contents |
Module 6: Containment
Description | Design | Construction | Characterisation | Modelling | Results | Conclusions
DNase Agar Assay
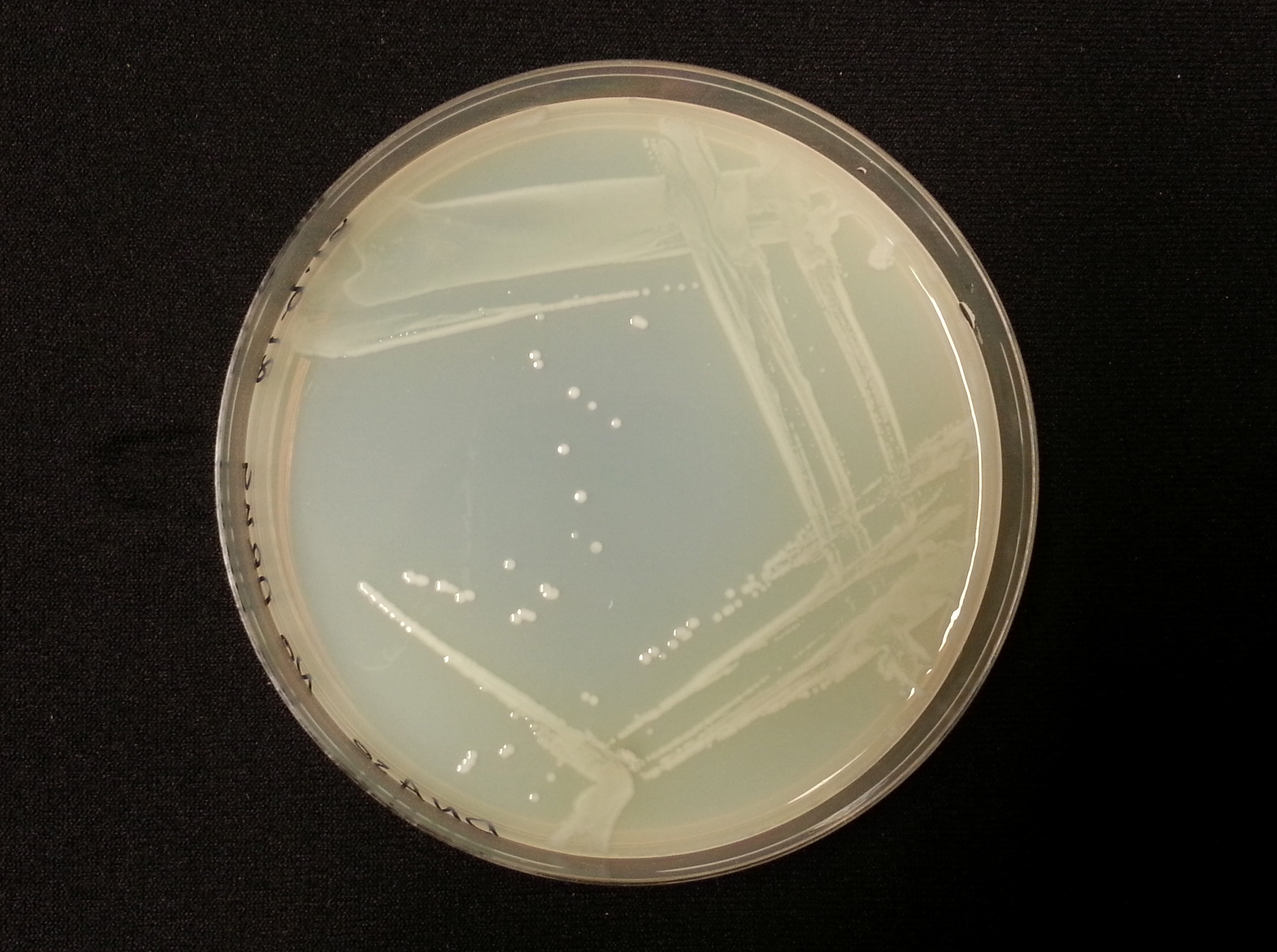
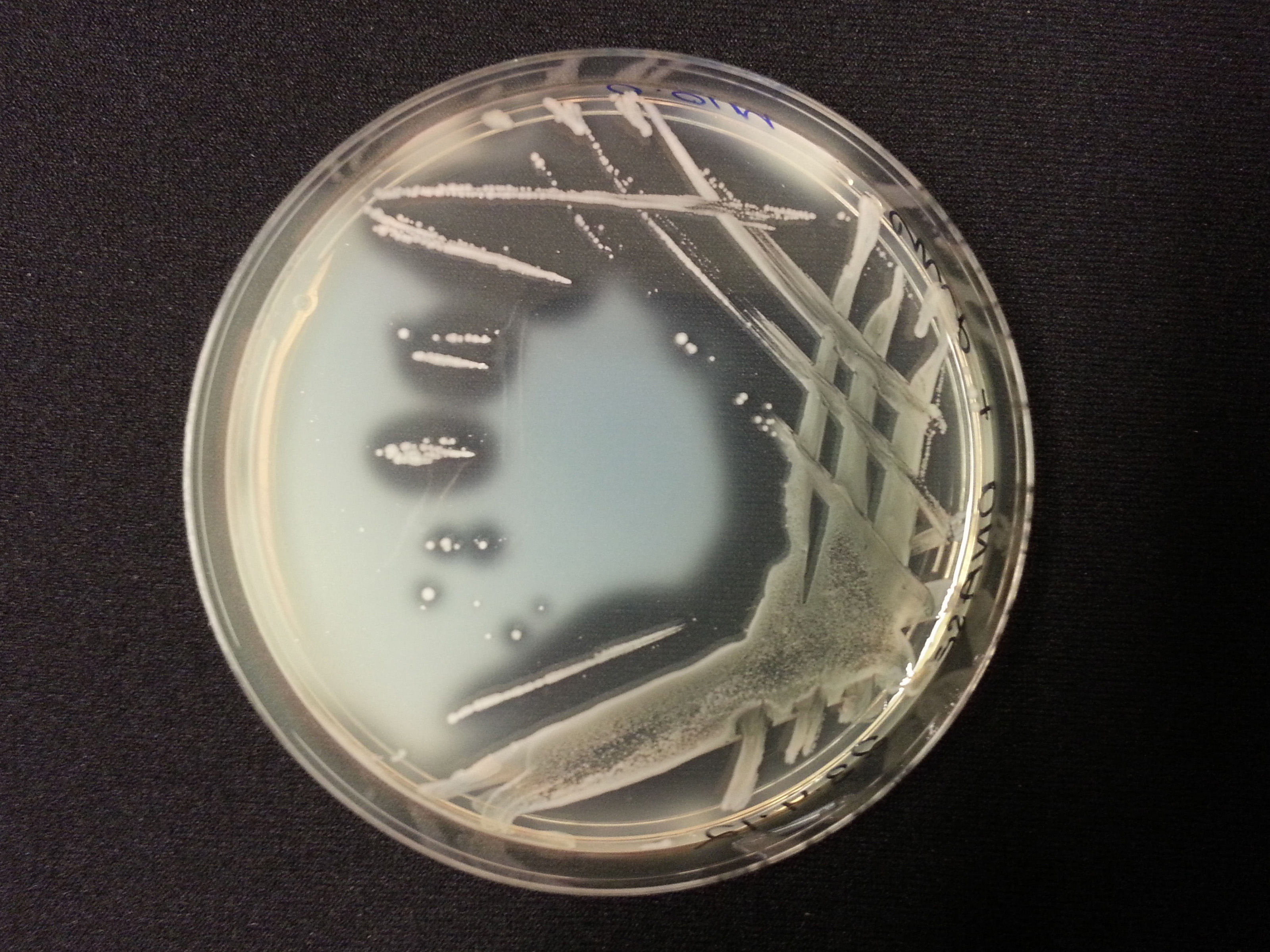
The following table shows the results we obtained from the DNase agar test. DNase agar contains DNA, which our periplasmic nuclease digests. This digestion is observed by adding hydrochloric acid to the agar plates, which causes the remaining DNA to precipitate, causing 'cloudiness' to the DNase agar.
As seen in our DNase agar plate, there is a clear halo surrounding our nuclease transformed cells, indicating a DNA-free zone where the nuclease has digested the DNA in the agar. No such halo is present in our untransformed cells. This indicates that our transformation has been successful, and that BBa_K729004 is working as expected, producing periplasmic nuclease that is capable of digesting extracellular genetic material.
| Control | BBa_K729019 |
|---|---|
 | 
|
Colony Halo size Assay
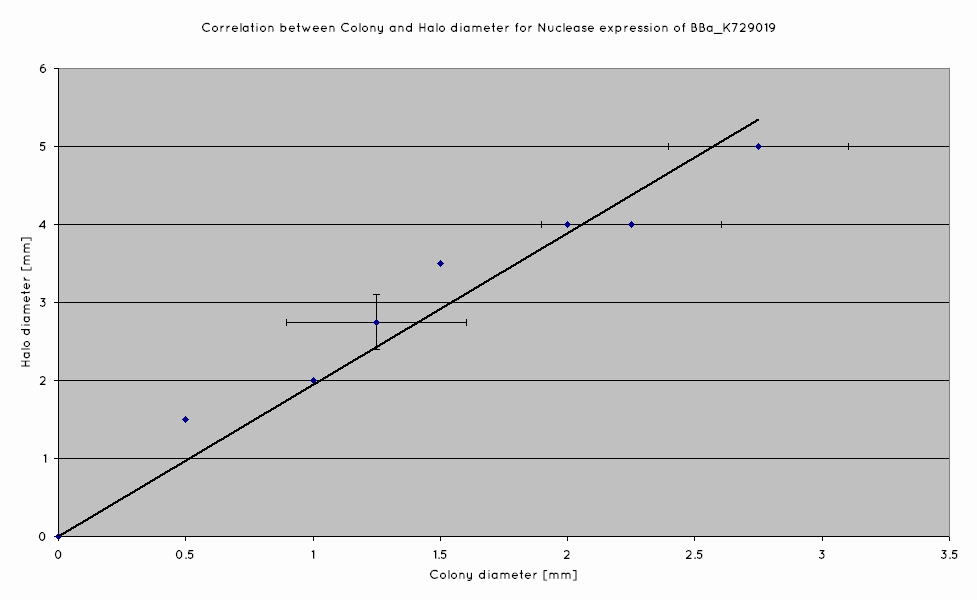
A separate DNase agar assay was also done, in order to determine if there was any correlation between colony size and nuclease production of our nuclease BioBrick (BBa_K729019). DNase agar was plated out with nuclease transformed W3110, at a concentration to produce single colonies on the plates. Colony sizes were measured, as wer the DNA-free zones surrounding the colonies.
We have determined that a linear correlation exists between colony size and halo diameter, hence suggesting that cells produce nuclease even as they grow.
Nuclease positive cells
| Time | Sample 1(Abs) | Sample 2 (Abs) |
|---|---|---|
| 0 | 0.001 | 0.001 |
| 1 | 0.004 | 0.006 |
| 2 | 0.006 | 0.009 |
| 3 | 0.009 | 0.016 |
| 4 | 0.24 | 0.035 |
| 5 | 0.053 | 0.076 |
| 6 | 0.084 | 0.116 |
| 7 | 0.183 | 0.217 |
| 8 | 0.299 | 0.479 |
| 9 | 0.684 | 0.918 |
| 10 | 0.801 | 1.499 |
| 11 | 0.913 | 1.804 |
| 12 | 1.022 | 2.033 |
Nuclease negative cells
| Time | Sample 1(Abs) | Sample 2 (Abs) |
|---|---|---|
| 0 | 0.001 | 0.001 |
| 1 | 0.001 | 0.001 |
| 2 | 0.009 | 0.005 |
| 3 | 0.026 | 0.014 |
| 4 | 0.034 | 0.026 |
| 5 | 0.068 | 0.057 |
| 6 | 0.092 | 0.088 |
| 7 | 0.179 | 0.184 |
| 8 | 0.306 | 0.297 |
| 9 | 0.666 | 0.678 |
| 10 | 0.777 | 0.799 |
| 11 | 0.924 | 0.955 |
| 12 | 1.073 | 1.099 |
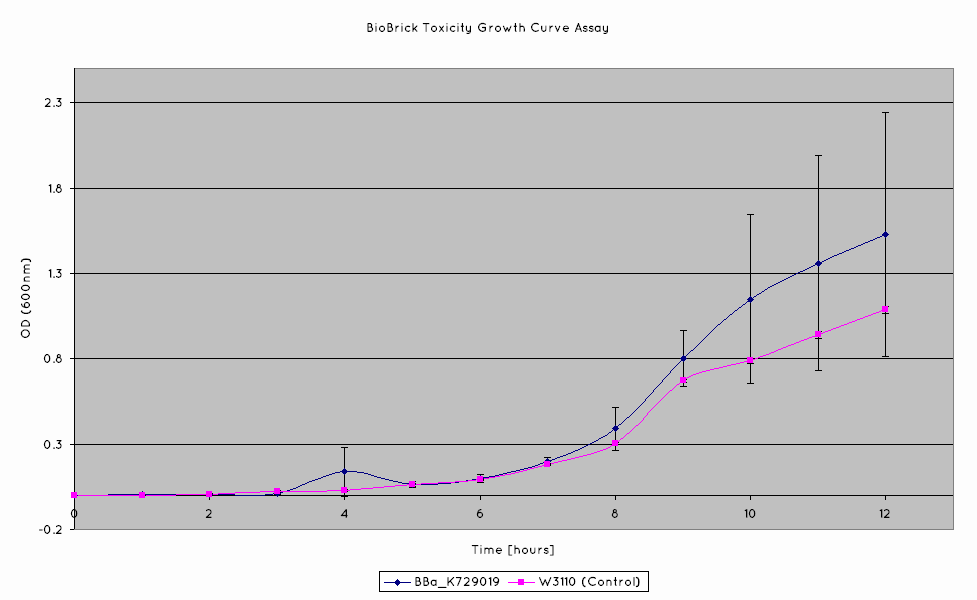
Growth Unaffected by Periplasmic Nuclease Expression
A growth curve assay was done on our nuclease transformed cells in order to determine if there was sufficient intracellular production of nuclease to adversely affect the viability of our cells. The cells were grown in shake flasks, and their optical density at 600nm was measured.
Our results indicate that the nuclease BioBrick does not negatively affect the growth of our cells. The growth rates obtained from our nuclease transformed cells are comparable to those of the untransformed W3110 cell line.
 "
"