Team:University College London/Module 1/Modelling
From 2012.igem.org
Erinoerton (Talk | contribs) (→Modelling) |
Erinoerton (Talk | contribs) (→References) |
||
| (32 intermediate revisions not shown) | |||
| Line 6: | Line 6: | ||
== Modelling == | == Modelling == | ||
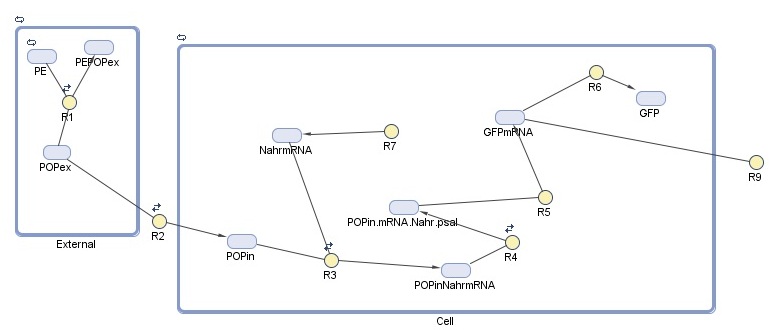
| - | As our detection module is so closely tied up with our degradation | + | As our detection module is so closely tied up with our degradation and aggregation modules, our cell model for this module examines the hypothetical expression of GFP on detection of POPs. This allows us to assess the efficacy of detection as a stand-alone module before we look at its performance when incorporated into our degradation system. |
[[File:detectionnet.jpg]] | [[File:detectionnet.jpg]] | ||
| - | + | Our cell model for detection was created in SimBiology. This MathWorks software is a MATLAB extension that allows specification of the species, reactions, and compartments that make up a system - in our case, the cell and the external environment - and to run simulations on the specified system. For this module, we are interested in detection response, which we represent here as GFP production. The model is composed of two 'compartments', which in SimBiology represent separate reaction systems: | |
| - | |||
| - | + | 1. ''''External'''': in this compartment we see the association, adherence, and dissociation of persistent organic pollutants (POPs) from polyethylene (PE) (R1). We rely on the POPs to induce our degradation system, increasing (10<sup>5</sup> to 10<sup>6</sup> the specificity to our system.) | |
| + | |||
| + | |||
| + | 2. ''''Cell'''': NahR is a constitutively produced mRNA product (R7). When POP diffuses into the cell (R2), it forms a complex with NahR (R3) which then binds to the pSal promoter (R4) to induce production of GFP (R5, R6). GFP is expressed in the cell or degrades (R9). | ||
==Species == | ==Species == | ||
| + | |||
{| class="bigtable" | {| class="bigtable" | ||
|- | |- | ||
| - | ! Species!! Initial value (molecules) !! Notes | + | ! Species!! Initial value (molecules) !! Notes & Assumptions |
|- | |- | ||
| PE|| 0.044 || Polyethylene found in North Pacific Gyre (value per cubic metre)<sup>1,2</sup> | | PE|| 0.044 || Polyethylene found in North Pacific Gyre (value per cubic metre)<sup>1,2</sup> | ||
| Line 26: | Line 29: | ||
| POPex || 0.0 || Persistent organic pollutants (ex = extracellular) that are not adhered to plastic surface | | POPex || 0.0 || Persistent organic pollutants (ex = extracellular) that are not adhered to plastic surface | ||
|- | |- | ||
| - | | PEPOPex || 9.24E-5 || Persistent organic pollutants (ex = extracellular) that are adhered to the plastic surface<sup> | + | | PEPOPex || 9.24E-5 || Persistent organic pollutants (ex = extracellular) that are adhered to the plastic surface<sup>6</sup> |
|- | |- | ||
| POPin || 0.5 || Persistent organic pollutants (in = intracellular) assumed from <i>E. coli</i> membrane permeability <sup>4</sup> | | POPin || 0.5 || Persistent organic pollutants (in = intracellular) assumed from <i>E. coli</i> membrane permeability <sup>4</sup> | ||
| Line 36: | Line 39: | ||
| POPinNahRpSal || 0.0 || Complex of the above molecule and pSal (promoter that induces laccase transcription) | | POPinNahRpSal || 0.0 || Complex of the above molecule and pSal (promoter that induces laccase transcription) | ||
|- | |- | ||
| - | | GFP || 0.0 || | + | | GFP || 0.0 || Green fluorescent protein |
|- | |- | ||
| GFPmRNA || 0.0 || GFP mRNA product | | GFPmRNA || 0.0 || GFP mRNA product | ||
| - | |||
| - | |||
| - | |||
| - | |||
|} | |} | ||
| Line 49: | Line 48: | ||
{| class="bigtable" | {| class="bigtable" | ||
|- | |- | ||
| - | ! Number !! Reaction !! Reaction rate (molecules/sec) !! Notes | + | ! Number !! Reaction !! Reaction rate (molecules/sec) !! Notes & Assumptions |
|- | |- | ||
| - | | R1 || PE + POPex ↔ PEPOPex || Forward: | + | | R1 || PE + POPex ↔ PEPOPex || Forward: 10000 <br /> Backward: 1 || Pops have 10000 to 100000 times greater tendency to adhere to plastic than float free in the ocean<sup>5</sup> |
|- | |- | ||
| - | | R2 || POPex ↔ POPin || Forward: 0.6 <br /> Backward: 0.4 || | + | | R2 || POPex ↔ POPin || Forward: 0.6 <br /> Backward: 0.4 || Rate is based on membrane permeability<sup>4</sup> and diffusion gradient |
|- | |- | ||
| - | | R3 || POPin + mRNA.Nahr | + | | R3 || POPin + mRNA.Nahr ↔ POPin.mRNA.Nahr || Forward: 1 <br /> Backward: 0.0001 || Based on the assumption that the chemical structure/size of POPs is similar to salycilate<sup>6</sup>. Salycilate binds to the NahR mRNA product, which complex then binds to the pSal promoter. |
|- | |- | ||
| - | | | + | | R4 || POPinmRNANahr ↔ POPinmRNANahr.Psal || Forward: 78200 <br /> Backward: 0.191 <sup>9</sup> || NahR to pSal binding based on the assumption that POP-NahR binding has no effect on NahR-pSal binding |
|- | |- | ||
| - | | | + | | R5 || POPexmRNANahr.Psal → GFP.mRNA|| 0.11 || Transcription rate of GFP in molecules/sec (for GFP size 720bp<sup>10</sup>, transcription rate in E.coli 80bp/sec<sup>8</sup>) |
|- | |- | ||
| - | | | + | | R6 || GFP.mRNA → GFP|| 0.084 || Translation rate of GFP in molecules/sec (for GFP size 240aa<sup>10</sup>, translation rate in E.coli 20aa/sec<sup>8</sup>) |
|- | |- | ||
| - | | | + | | R7 || 0 → mRNA.Nahr || Forward: 0.088 <br /> Backward: 0.6 || Transcription rate of NahR in molecules/sec (for NahR size 909 bp<sup>7</sup>, transcription rate in E.coli 80bp/sec<sup>8</sup>) under constitutive promoter control |
|- | |- | ||
| - | | | + | | R9 || GFP.mRNA → 0 || 0.03 || Degradation rate of GFP mRNA product<sup>11</sup> must be taken into account due to suboptimal conditions |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
|} | |} | ||
== Results == | == Results == | ||
| - | + | [[File:gfpgraph.png]] | |
| - | + | ||
| - | [[File: | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
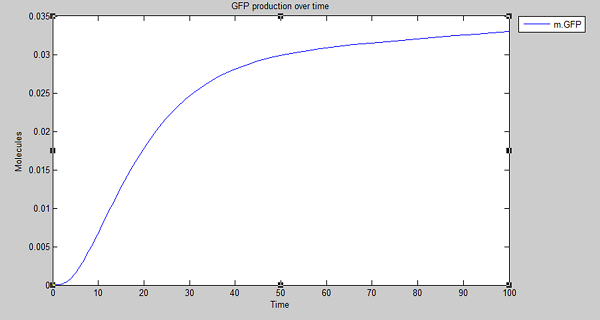
| - | + | Results from model simulations show that GFP is expressed upon detection of persistent organic pollutants, such as polyethylene, in line with expectations. The rate of increase in fluorescence levels eventually decreases, with a maximal asymptotic expression being reached. With this in mind and satisfied that degradation is working as expected ''in silico'', we extended the model to create the [[Team:University_College_London/Module_3/Modelling|cell model for degradation]] and show how polyethylene is degraded by Laccase proteins and how sensitive it is to changes within biochemical rates. | |
== References == | == References == | ||
| Line 90: | Line 79: | ||
2. Andrady AL (2011) Microplastics in the marine environment. <i>Marine Pollution Bulletin</i> 62: 1596-1605 | 2. Andrady AL (2011) Microplastics in the marine environment. <i>Marine Pollution Bulletin</i> 62: 1596-1605 | ||
| - | + | 4. Kay J, Koivusalo M, Ma X, Wohland T, Grinstein S (2012) Phosphatidylserine Dynamics in Cellular Membranes. <i>Molecular Biology of the Cell</i> | |
| - | + | ||
| - | 4. | + | |
5. Mato Y, Isobe T, Takada H, Kanehiro H, Ohtake C, Kaminuma T (2001) Plastic Resin Pellets as a Transport Medium for Toxic Chemicals in the Marine Environment. <i>Environ. Sci. Technol.</i> 35: 318-324 | 5. Mato Y, Isobe T, Takada H, Kanehiro H, Ohtake C, Kaminuma T (2001) Plastic Resin Pellets as a Transport Medium for Toxic Chemicals in the Marine Environment. <i>Environ. Sci. Technol.</i> 35: 318-324 | ||
| Line 104: | Line 91: | ||
9. Park H, Lim W, Shin H (2005) In vitro binding of purified NahR regulatory protein with promoter Psal. <i>Biochimica et Biophysica Acta</i> 1775: 247-255 | 9. Park H, Lim W, Shin H (2005) In vitro binding of purified NahR regulatory protein with promoter Psal. <i>Biochimica et Biophysica Acta</i> 1775: 247-255 | ||
| - | 10. | + | 10. http://partsregistry.org/wiki/index.php?title=Part:BBa_I13522 |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | 11. Kushner S (2002) mRNA Decay in <i>Escherichia coli</i> Comes of Age. <i>J Bacteriol.</i> 184: 4658-4665 | |
| - | |||
{{:Team:University_College_London/templates/foot}} | {{:Team:University_College_London/templates/foot}} | ||
Latest revision as of 13:33, 26 September 2012
Contents |
Module 1: Detection
Description | Design | Construction | Characterisation | Modelling | Conclusions
Modelling
As our detection module is so closely tied up with our degradation and aggregation modules, our cell model for this module examines the hypothetical expression of GFP on detection of POPs. This allows us to assess the efficacy of detection as a stand-alone module before we look at its performance when incorporated into our degradation system.
Our cell model for detection was created in SimBiology. This MathWorks software is a MATLAB extension that allows specification of the species, reactions, and compartments that make up a system - in our case, the cell and the external environment - and to run simulations on the specified system. For this module, we are interested in detection response, which we represent here as GFP production. The model is composed of two 'compartments', which in SimBiology represent separate reaction systems:
1. 'External': in this compartment we see the association, adherence, and dissociation of persistent organic pollutants (POPs) from polyethylene (PE) (R1). We rely on the POPs to induce our degradation system, increasing (105 to 106 the specificity to our system.)
2. 'Cell': NahR is a constitutively produced mRNA product (R7). When POP diffuses into the cell (R2), it forms a complex with NahR (R3) which then binds to the pSal promoter (R4) to induce production of GFP (R5, R6). GFP is expressed in the cell or degrades (R9).
Species
| Species | Initial value (molecules) | Notes & Assumptions |
|---|---|---|
| PE | 0.044 | Polyethylene found in North Pacific Gyre (value per cubic metre)1,2 |
| POPex | 0.0 | Persistent organic pollutants (ex = extracellular) that are not adhered to plastic surface |
| PEPOPex | 9.24E-5 | Persistent organic pollutants (ex = extracellular) that are adhered to the plastic surface6 |
| POPin | 0.5 | Persistent organic pollutants (in = intracellular) assumed from E. coli membrane permeability 4 |
| mRNANahR | 0.0 | NahR mRNA product |
| POPinNahR | 0.0 | Complex of the above two molecules |
| POPinNahRpSal | 0.0 | Complex of the above molecule and pSal (promoter that induces laccase transcription) |
| GFP | 0.0 | Green fluorescent protein |
| GFPmRNA | 0.0 | GFP mRNA product |
Reactions taking place in the model
| Number | Reaction | Reaction rate (molecules/sec) | Notes & Assumptions |
|---|---|---|---|
| R1 | PE + POPex ↔ PEPOPex | Forward: 10000 Backward: 1 | Pops have 10000 to 100000 times greater tendency to adhere to plastic than float free in the ocean5 |
| R2 | POPex ↔ POPin | Forward: 0.6 Backward: 0.4 | Rate is based on membrane permeability4 and diffusion gradient |
| R3 | POPin + mRNA.Nahr ↔ POPin.mRNA.Nahr | Forward: 1 Backward: 0.0001 | Based on the assumption that the chemical structure/size of POPs is similar to salycilate6. Salycilate binds to the NahR mRNA product, which complex then binds to the pSal promoter. |
| R4 | POPinmRNANahr ↔ POPinmRNANahr.Psal | Forward: 78200 Backward: 0.191 9 | NahR to pSal binding based on the assumption that POP-NahR binding has no effect on NahR-pSal binding |
| R5 | POPexmRNANahr.Psal → GFP.mRNA | 0.11 | Transcription rate of GFP in molecules/sec (for GFP size 720bp10, transcription rate in E.coli 80bp/sec8) |
| R6 | GFP.mRNA → GFP | 0.084 | Translation rate of GFP in molecules/sec (for GFP size 240aa10, translation rate in E.coli 20aa/sec8) |
| R7 | 0 → mRNA.Nahr | Forward: 0.088 Backward: 0.6 | Transcription rate of NahR in molecules/sec (for NahR size 909 bp7, transcription rate in E.coli 80bp/sec8) under constitutive promoter control |
| R9 | GFP.mRNA → 0 | 0.03 | Degradation rate of GFP mRNA product11 must be taken into account due to suboptimal conditions |
Results
Results from model simulations show that GFP is expressed upon detection of persistent organic pollutants, such as polyethylene, in line with expectations. The rate of increase in fluorescence levels eventually decreases, with a maximal asymptotic expression being reached. With this in mind and satisfied that degradation is working as expected in silico, we extended the model to create the cell model for degradation and show how polyethylene is degraded by Laccase proteins and how sensitive it is to changes within biochemical rates.
References
1. Goldstein M, Rosenberg M, Cheng L (2012) Increased oceanic microplastic debris enhances oviposition in an endemic pelagic insect, Biology Letters 10.1098
2. Andrady AL (2011) Microplastics in the marine environment. Marine Pollution Bulletin 62: 1596-1605
4. Kay J, Koivusalo M, Ma X, Wohland T, Grinstein S (2012) Phosphatidylserine Dynamics in Cellular Membranes. Molecular Biology of the Cell
5. Mato Y, Isobe T, Takada H, Kanehiro H, Ohtake C, Kaminuma T (2001) Plastic Resin Pellets as a Transport Medium for Toxic Chemicals in the Marine Environment. Environ. Sci. Technol. 35: 318-324
6. https://2011.igem.org/Team:Peking_S/project/wire/harvest
7. http://www.xbase.ac.uk/genome/azoarcus-sp-bh72/NC_008702/azo2419;nahR1/viewer
8. http://kirschner.med.harvard.edu/files/bionumbers/fundamentalBioNumbersHandout.pdf
9. Park H, Lim W, Shin H (2005) In vitro binding of purified NahR regulatory protein with promoter Psal. Biochimica et Biophysica Acta 1775: 247-255
10. http://partsregistry.org/wiki/index.php?title=Part:BBa_I13522
11. Kushner S (2002) mRNA Decay in Escherichia coli Comes of Age. J Bacteriol. 184: 4658-4665
 "
"