Team:USP-UNESP-Brazil/Plasmid Plug n Play/Results
From 2012.igem.org
Andresochoa (Talk | contribs) |
Andresochoa (Talk | contribs) |
||
| Line 19: | Line 19: | ||
<h1 id="''In vivo'' assay ">''In vivo'' assay </h1> | <h1 id="''In vivo'' assay ">''In vivo'' assay </h1> | ||
| - | We proved that we can circularize a fragment of DNA (kanamycin resistance gene) flanked by a loxP and a lox66 sites ''in vitro'', so we decided to test one of the Plug&Play prototypes (T7-lox71-Cre-pSB4A5). This plasmid produces a low copy number and has a | + | We proved that we can circularize a fragment of DNA (kanamycin resistance gene) flanked by a loxP and a lox66 sites ''in vitro'', so we decided to test one of the Plug&Play prototypes (T7-lox71-Cre-pSB4A5). This plasmid produces a low copy number and has a gene that confers resistance to ampicillin. The Cre recombinase is under the control of the T7 promoter, the target gene (in this case the kanamycin resistance gene) will be inserted in the lox71 site upstream the Cre recombinase gene. When the gene is inserted it will be controlled by the same T7 promoter. This plasmid is part of the six prototypes we are developing, the comparison of these plasmids will identify the best system for the developing of this technology. |
| + | For this experiment we needed a bacteria lineage with the Plug&Play prototype. So we transformed electrocompetent ''E. coli''(Bl21(DE3)) cells with 1ul(80ng) of the Plug&Play prototype. Then, we prepared electrocompetent cells using this transformed bacteria, producing a Plug&Play Machine ready to receive the kanamycin resistance gene. | ||
| - | + | We transformed electrocompetent Plug&Play Machine cells with a gradient of our PCR-product (kanamycin resistance gene flanked by a loxP and a lox66): 10ng, 100ng and 1000ng. This concentration were chosen using the mathematical model that the group already had. After transformation the cells (50ul) were left for 40 min in LB medium without antibiotic for recovering from the transformation stress. This LB medium with the cells (1mL medium) was equally divided in three LB plates. One plate had ampicillin and IPTG, the second had kanamycin and IPTG and the last one had ampicillin, kanamycin and IPTG. The plates were left incubating for 15h at 37°C. As a control, the pET15b plasmid was used for a independent transformation of the ''E. coli''(Bl21(DE3)) lineage and plated in the same three types of plates. The pET15b plasmid has a gene that confers resistance to ampicillin. | |
| - | We found | + | We found |
| - | |||
| - | |||
{{:Team:USP-UNESP-Brazil/Templates/Foot}} | {{:Team:USP-UNESP-Brazil/Templates/Foot}} | ||
Revision as of 14:03, 26 September 2012
 Introduction
Introduction Project Overview
Project Overview Plasmid Plug&Play
Plasmid Plug&Play Associative Memory
Associative MemoryNetwork
 Extras
ExtrasIn vitro assay
This experiment was made to test if the loxP and the lox66 were working properly, it also allowed us to measure the Cre concentration needed for an in vitro recombination reaction. The particularity of lox66 is that it has an altered sequence at the end of it's left arm when compared to loxP (natural recombination site from P1 Bacteriophage). This sequence variation reduces affinity of the Cre recombinase for the arm.
Based on the report made by the igem2010 UT-Tokyo team and some papers (e.g http://www.ncbi.nlm.nih.gov/pmc/articles/PMC137435/), the lox66 (BBa_I718016) from the registry was wrong, it had a gg instead a cg in its left arm. This part was corrected by the iGEM11_Tokyo_Tech team (BBa_K649206) and by the iGEM11_WITS_CSIR_SA team (BBa_K537019), but no DNA was available in the registry. Anyway, we needed to synthesized it and test it as part of our PCR-primers, we used the proper sequence described by http://www.ncbi.nlm.nih.gov/pmc/articles/PMC137435/.
We designed two primers, one containing the lox66 and one containing the loxP sequences, these primers amplified the ORF from the kanamycin resistance gene, flanked upstream by the loxP and downstream by the lox66, using PCR. These sites should be recognized by the Cre recombinase (from NEB company), which could circularized our linear PCR product. This is important because we don't want it to be degraded when inserted in the bacteria. This In vitro assay was a test for a posterior In vivo assay, where we expect that this process happens inside the E. coli using a Cre recombinase enzyme expressed by the same bacteria.
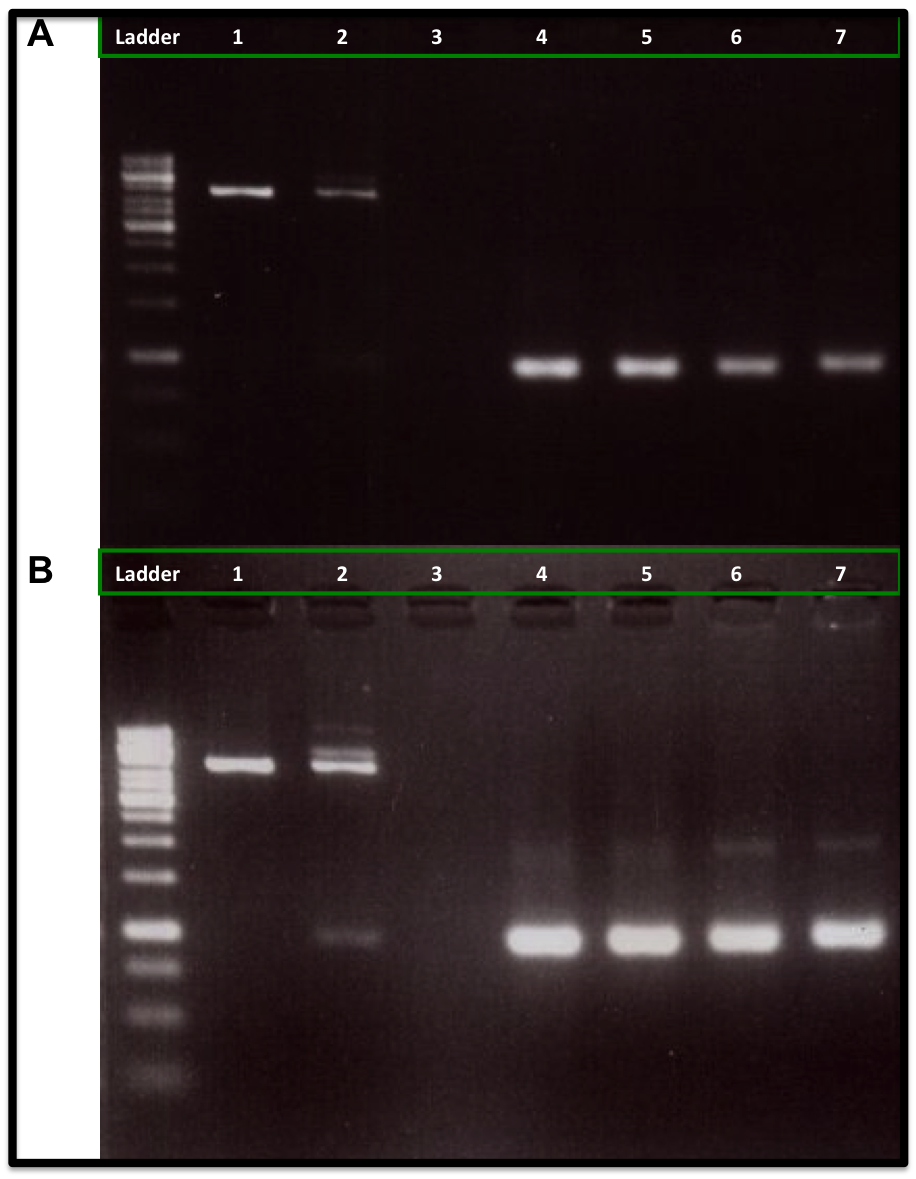
Our experiment showed that 5U and 10U of Cre recombinase produced a reduction of linear DNA (Kanamycin resistance gene flanked with loxP and lox66) when compare to 1U of Cre recombinase and to the control DNA (No Cre recombinase added), as is showed in the figure A. It was also observed an increase of the DNA plasmid form (upper band at 2kb), as is showed in figure B. We also used a control DNA substrate supplied in the NEB recombinase kit. The same amount of PCR-product was applied to each lane (250ng).
The conclusion was that we can use this loxP-lox66 mechanism in our design and we will need at least 5U of Cre recombinase for any in vitro experiment.
In vivo assay
We proved that we can circularize a fragment of DNA (kanamycin resistance gene) flanked by a loxP and a lox66 sites in vitro, so we decided to test one of the Plug&Play prototypes (T7-lox71-Cre-pSB4A5). This plasmid produces a low copy number and has a gene that confers resistance to ampicillin. The Cre recombinase is under the control of the T7 promoter, the target gene (in this case the kanamycin resistance gene) will be inserted in the lox71 site upstream the Cre recombinase gene. When the gene is inserted it will be controlled by the same T7 promoter. This plasmid is part of the six prototypes we are developing, the comparison of these plasmids will identify the best system for the developing of this technology.
For this experiment we needed a bacteria lineage with the Plug&Play prototype. So we transformed electrocompetent E. coli(Bl21(DE3)) cells with 1ul(80ng) of the Plug&Play prototype. Then, we prepared electrocompetent cells using this transformed bacteria, producing a Plug&Play Machine ready to receive the kanamycin resistance gene.
We transformed electrocompetent Plug&Play Machine cells with a gradient of our PCR-product (kanamycin resistance gene flanked by a loxP and a lox66): 10ng, 100ng and 1000ng. This concentration were chosen using the mathematical model that the group already had. After transformation the cells (50ul) were left for 40 min in LB medium without antibiotic for recovering from the transformation stress. This LB medium with the cells (1mL medium) was equally divided in three LB plates. One plate had ampicillin and IPTG, the second had kanamycin and IPTG and the last one had ampicillin, kanamycin and IPTG. The plates were left incubating for 15h at 37°C. As a control, the pET15b plasmid was used for a independent transformation of the E. coli(Bl21(DE3)) lineage and plated in the same three types of plates. The pET15b plasmid has a gene that confers resistance to ampicillin.
We found
 "
"