Team:Tianjin/Safety
From 2012.igem.org
| (3 intermediate revisions not shown) | |||
| Line 79: | Line 79: | ||
<div class="menu_children"> | <div class="menu_children"> | ||
<a href="#Synthetic_Biology">Synthetic Biology</a> | <a href="#Synthetic_Biology">Synthetic Biology</a> | ||
| + | </div> | ||
| + | <div class="menu_children"> | ||
| + | <a href="#Regulation_with_density:_XMU_gold_medal.EF.BC.8CAdvance_to_Championship">Regulation with density: XMU gold medal,Advance to Championship</a> | ||
| + | </div> | ||
| + | <div class="menu_children"> | ||
| + | <a href="#Regulation_with_Light">Regulation with Light</a> | ||
| + | </div> | ||
| + | <div class="menu_children"> | ||
| + | <a href="#Regulation_with_temperature.2C_UTP-Panama.2C_Gold_medal">Regulation with temperature, UTP-Panama, Gold medal</a> | ||
| + | </div> | ||
| + | <div class="menu_children"> | ||
| + | <a href="#Regulation_with_Quorum-Sensing.2C_THU.2CGold_medal">Regulation with Quorum-Sensing, THU,Gold medal</a> | ||
</div> | </div> | ||
<div class="menu_children"> | <div class="menu_children"> | ||
<a href="#References">References</a> | <a href="#References">References</a> | ||
</div> | </div> | ||
| + | |||
</div> | </div> | ||
<div class="menu_head"> | <div class="menu_head"> | ||
| Line 178: | Line 191: | ||
Various methods of regulation are provide.Followings are the typical ones. | Various methods of regulation are provide.Followings are the typical ones. | ||
=Regulation with density: XMU gold medal,Advance to Championship= | =Regulation with density: XMU gold medal,Advance to Championship= | ||
| + | [[file:TJU1201.jpg|center]] | ||
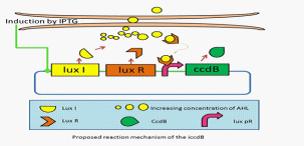
They have developed a series of devices which program a bacteria population to maintain at different cell densities. They have designed and characterized the genetic circuit to establish a bacterial ‘population-control’ device in E. coli based on the well-known quorum-sensing system from Vibrio fischeri, which autonomously regulates the density of an E. coli population. The cell density however is influenced by the expression levels of a killer gene (ccdB) in our device. As such, we have successfully controlled the expression levels of ccdB by using RBSes of different strength and mutated luxR promoters (lux pr). They are working on builting up a database for a series of mutation sites and RBSes corresponding to different steady-state cell densities. An artificial neural network will be built to model and predict the cell density of an E. coli population. This work can serve as a foundation for future advances involving fermentation industry and information processing. | They have developed a series of devices which program a bacteria population to maintain at different cell densities. They have designed and characterized the genetic circuit to establish a bacterial ‘population-control’ device in E. coli based on the well-known quorum-sensing system from Vibrio fischeri, which autonomously regulates the density of an E. coli population. The cell density however is influenced by the expression levels of a killer gene (ccdB) in our device. As such, we have successfully controlled the expression levels of ccdB by using RBSes of different strength and mutated luxR promoters (lux pr). They are working on builting up a database for a series of mutation sites and RBSes corresponding to different steady-state cell densities. An artificial neural network will be built to model and predict the cell density of an E. coli population. This work can serve as a foundation for future advances involving fermentation industry and information processing. | ||
| Line 187: | Line 201: | ||
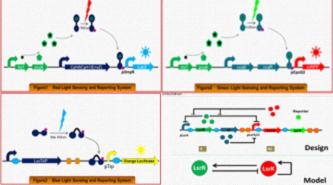
In the second part, They came up with the idea of “colorful E.film”. In hope to create a colorful film, They will construct three E.coli strains which can produce and secrete three primary colors respectively in the presence of the three primary lights. | In the second part, They came up with the idea of “colorful E.film”. In hope to create a colorful film, They will construct three E.coli strains which can produce and secrete three primary colors respectively in the presence of the three primary lights. | ||
| + | [[file:TJU1202.jpg|center]] | ||
===UC Davis, gold medal=== | ===UC Davis, gold medal=== | ||
| Line 197: | Line 212: | ||
They dedicate in pursuing the goal of the construction of a biological oscillator, which they call 'ECHO', the abbreviation of 'the ''E. coli'' Homochronous Oscillator'. | They dedicate in pursuing the goal of the construction of a biological oscillator, which they call 'ECHO', the abbreviation of 'the ''E. coli'' Homochronous Oscillator'. | ||
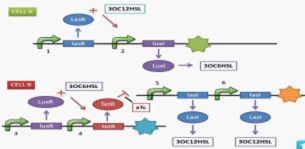
With two Escherichia coli populations expressing gene one after another, they give red and green fluorescent light alternately. E.coli populations communicate bi-directionally by a class of signaling molecules involves in bacteria quorum sensing, that is, N-Acyl homoserine lactones (AHL), to regulate the gene expression of each other. By engineering their gene circuits, two groups (name as CELL-A and CELL-B) will form a network, with B inducing A and A restricting B, thus able to realize oscillation. A mechanism is set up to change the rate of an AHL expression, allowing us to control the period and the phase of the oscillatory cycle. | With two Escherichia coli populations expressing gene one after another, they give red and green fluorescent light alternately. E.coli populations communicate bi-directionally by a class of signaling molecules involves in bacteria quorum sensing, that is, N-Acyl homoserine lactones (AHL), to regulate the gene expression of each other. By engineering their gene circuits, two groups (name as CELL-A and CELL-B) will form a network, with B inducing A and A restricting B, thus able to realize oscillation. A mechanism is set up to change the rate of an AHL expression, allowing us to control the period and the phase of the oscillatory cycle. | ||
| + | [[file:TJU1203.jpg|center]] | ||
As a translational regulatory tool, our device achieves more precise tuning of protein expression when compared with transcriptional tools. The controlling elements we use in this device are codons and tRNAs, the major participants of translation process. Thus manipulating these elements exert direct effects on protein biosynthesis. We just try to summarize something useful for more teams to refer to. | As a translational regulatory tool, our device achieves more precise tuning of protein expression when compared with transcriptional tools. The controlling elements we use in this device are codons and tRNAs, the major participants of translation process. Thus manipulating these elements exert direct effects on protein biosynthesis. We just try to summarize something useful for more teams to refer to. | ||
Latest revision as of 03:57, 27 September 2012

Genetic pollution refers to the unintended or uncontrolled gene flow of native species gene pool. In 21th century, the recombinant organisms begin to spread into the environment extensively. Admittedly, scientists and international groups have already started to seriously consider the safety of transgenosis, but there are still a lot of limitations and a lack of long-term data. Although genetic engineering can bring huge benefits to us, but the potential threats it cause can never be ignored. Like DDT, which won a Nobel Prize in the year when it was created, but caused great injury to both human beings and the environment after 50 years. We can't stop this kind of things totally, but at least we can make efforts to prevent it. Considering the specialty of our program, it can be creatively used in the career of the gene pollution prevention, we will show the possibilities and examples in the third part. We divided our handbook into three parts:
Operation
Laboratory Practices Followed By Our Team
- 1. Always wear appropriate personal protective equipment. Feet and legs should be covered; sandals and open-toed shoes should not be worn in laboratories. Wear appropriate gloves while handling infectious or toxic materials and animals. Do not wear lab coats, gloves or other personal protective equipment outside the laboratory. Change gloves when contaminated, and dispose of used gloves with other contaminated laboratory waste.
- 2. Do not eat, drink, smoke, handle contact lenses, apply cosmetics, or store food for human consumption in the laboratory.
- 3. Wash your hands after working with potentially hazardous materials and before leaving the laboratory.
- 4. Follow the institutional policies regarding safe handling of sharps (i.e., needles, scalpels, pipettes, and broken glassware). Be careful with needles and syringes. Use only when alternative methods are not feasible. Syringes, needles, Pasteur pipettes, etc, should be placed in rigid, leak-proof containers (Sharps Safe) and discarded following the waste rules.
- 5. Take care to minimize the creation of aerosols and/or splashes.
- 6.Decontaminate all work surfaces before and after your experiments, and immediately after any spill or splash of potentially infectious material with an appropriate disinfectant. Clean laboratory equipment routinely, even if it is not contaminated.
- 7. Decontaminate all potentially infectious materials before disposal.
- 8. Report any incidents that may result in exposure to infectious materials to appropriate personnel (e.g., laboratory supervisor, safety officer).
- 9. Know where the nearest eyewash, safety shower, and fire extinguisher are located. Know how to use them.
- 10. Insist upon good housekeeping in your laboratory.
- 11. Check for insects and rodents. Keep all areas clean.
- 12. Secure all gas cylinders.
- 13. Use a biological safety cabinet for handling infectious materials or materials requiring protection from contamination and a fume hood for toxic materials; mixed hazards need to be evaluated case by case.
- 14. Fume hoods should be used for laboratory activities that could result in chemical explosions or fires, for experiments involving toxic, hazardous or carcinogenic compounds, and use of strong acids and bases. 9Biological safety cabinets should not be used for this kind of work. Fume hoods are workstations, not storage cabinets. Vented storage areas may be located under the fume hood work area. However, these are not for storage of flammables.
- 15. Respect chemicals and radionuclides. Know their hazards and follow appropriate safety precautions. Chemical and radioactive waste must not be poured down the drain.
- 16. All equipment must be documented to be free of chemical, biological, and radiological contamination before repair work is done or before moving equipment for storage or elsewhere.
- 17. Never mouth pipette anything. Use mechanical pipetting devices only!
- 18. Close laboratory doors while experiments are in progress. Restrict access to laboratory.
- 19. Put liquid traps and in-line vacuum filters on all vacuum lines.
- 20. Minimize or contain all aerosol-producing activities, large-volume work, concentrated solutions or cultures. These activities include centrifugation (use safety cups), vortex missing (stopper tube), blending (use metal safety blender), sonication, grinding, opening containers of infectious material, inoculating culture flasks, inoculating animals, harvesting infectious materials from cultures or animals, and weighing or reconstructing toxic powders, etc.
- 21. Place biological safety cabinets in low-traffic areas and minimize activities that disrupt air flow in or around cabinet.
- 22. Decontaminate all work surfaces daily, and decontaminate all spills immediately.
- 23. Decontaminate (by autoclaving or chemical disinfection) all biologically contaminated materials – glassware, animal cages, laboratory equipment, etc. – before washing, reuse or disposal. Discard materials via proper waste stream.
- 24. Broken glassware and disposable pipettes (after decontamination) should be placed in a “Disposable Labware and Broken Glass Box” and discarded following the waste rules.
- 25. Place contaminated biological materials in covered, leak-proof containers before removing them from the laboratory.
- 26. Wash your hands after handling chemicals, infectious materials, animals, after removing gloves and before leaving the laboratory.
Social
- 1. Everyone should be prudent
DDT, the inventor of which won the Nobel Prize, was used as a pesticide at early time, but after 50 years we found out that it caused irreparable damage to human beings and environment. Therefore, it is necessary to slow the development of genetic engineering and focus on improving its basic research. We should make sure it won't cause side effects to the environment and human beings before its promotion.
- 2. Establish and improve the related laws and regulations, and strictly implement them
In the past two decades, Chinese government also put forward a lot of related laws and regulations. In 1993, the former State Science and Technology Commission issued a regulation called "genetic engineering safety management measures". In 1996 the Ministry of Agriculture issued the “Agricultural Biological Genetic Engineering Safety Management Implementation Approach". In May 23, 2001, the State Council announced “Agricultural Genetically Modified Organisms' (GMOs') Safety Management Regulations". On January 5, 2002, the Ministry of Agriculture announced “Safety Assessment of Agricultural GMO", "Measures for the Administration of the Import of Agricultural Transgenic Living Things", “Agricultural GMO Identity Management Approach ". These laws and regulations help organizations to stick to principles to prevent genetic pollution.
- 3. Improve the examination and approval system of gene engineering technology application
The system should require the genetic engineering technology pass the microbiology, plant and animal experiments, environmental experiments and human trials before put into application. We should prevent the abuse and industrialization or commercialization of genetic engineered product rashly. For example, some governments have made announcements to prohibit the production and sale of any crops with antibiotic resistance gene.
- 4. Carry out public science education
The lack of the biological safety awareness is one of the biggest reasons of the genetic pollution. The majority of Chinese people whose daily life are closely related to genetically modified food can't ever understand the genetic pollution. So it's necessary for us to carry out the popular science education so that more and more people can pay attention to the genetic engineering knowledge.
- 5. Implementation of a labelling system for genetically modified food
United Nations announce if we take people's health and the environment into account, every country has the right to restrict the import of genetically modified food. All genetically modified products in the shipment should be labeled, indicating “this product contains genetically modified material ". International Consumers Association believe that although it is uncertain that genetically modified food is unsafe, but in order to prevent potential hazard to human, we should take preventive measures and establish an identification system. The identification of GMOs can help consumers to make choices. Genetic pollution is really a big problem in the application of genetic engineering. But we must realize the great benefit of genetic engineering. It is likely to bring the best choice for us to solve the global food shortage. Therefore we cannot give up improving gene engineering technology for fear of a little trouble.
- 6. Risk assessment and management
Conduct the risk assessment and management of transgenic technique and analysis the adverse effects of its products in the trans-boundary movement process so that the importer can make choices easily.
Synthetic Biology
When it comes to our iGEM project this year, the regulation of gene expression systems can not only introduces a new way for protein-specific expression but also plays an important role in preventing genetic pollution. For example, if you want to transfer some exogenous genes that are harmful to the environment or human beings into cells, then biological safety is one thing we must consider about. To prevent harmful genes being expressed in uncontrolled cases, we must do something to prevent possible genetic pollution. And this year our project can do a lot on this problem. Our o-ribosome device allows translation only when the combination of the o-16s ribosome and o-RBS, so if we put the device at the upstream of the target genes, we can control the expression of target genes precisely. At the same time, RBS sequences are necessary in almost every cell,therefore an extensive application can be performed by our o-ribosomal. Not only can our project regulate gene expression and prevent genetic pollution, but also it will not consume any extra nutrients, and have no influence on the normal life activities of bacteria.On the other hand, different RBS sequences can influence the efficiency of the translation, so we can use a series of RBS sequences to regulate gene expression with a gradient rate. The possibility of genetic pollution can also be reduced in this accurate process.
Finally, the o-ribosomal devices can be used to establish a orthogonal system, in which cells have specific o-ribosomal devices instead of the original ones. Being different from other ordinary cells, they cannot be expressed in normal cells even if the target genes from orthogonal system spread out,which leads to a strong protection against genetic pollution. It is obvious that genetic pollution can be completely prevented in this orthogonal system. There were also several projects which were related to gene expression regulation in iGEM last year. For example, the SJTU-BioX-Shanghai team designed a set of Codon-Switches that regulate target protein biosynthesis (translation).In their rare-codon switch, the translation of the protein can be finely turned up/down with the control of rare tRNA amount, aaRS that charges the rare tRNA and rare codons. Besides they also made other two switches that could be turned on/off without background noise. One called the stop-codon switch was to use stop codon as the controlling element. The other one called the initial-codon switch was to use any codon but the original start codon to initiate translation. What's more, the projects of the BYU Provo team, USTC-China team, Peking-R team and some other teams were all related to gene expression regulation too. We believe that maybe the principles of these projects can also be used in the prevention to genetic pollution. So if you are interested in this, you can know more through their wiki.
As iGEM teams are from different countries which may have different regulations for biosaftey. So on the one hand, it's necessary to collect information and make standard biosafety rules for basic synthetic biology experiments. On the other hand, our simple biosafety handbook cannot be suitable for all teams. But we believe our work must be useful for many iGEM teams and other researchers.
The creature differs from each other due to the patterns of gene expression,because most genes differ in their level of the cell cycle.The activity of genes is also keyed to the functions of the cell;The expression of the protein can influence the whole cell.The main role in gene regulation is the level of transcription,either through signals originating within the cell itself or in response to external conditions.Many gene products are needed only on ocassion ,and transcription can be regulated in an on-off manner that enables such products to ve present only when external conditions demand them .Five main control points for gene expression include:
- Transcriptional regulation of the synthesis of RNA transcrips by controling initiation or termination.
- RNA processing or regulation through RNA splicing or alternative patterns of splicing.
- Translational control of polypeptide synthesis.
- Stability of mRNA,because mRNAs that persist in the cell have longer-lasting effects than those that are degraded rapidly.
- Posttranslational control,which includes a great variety of mechanisms that affect enzyme activity, activation,stability, and so on.
- DNA rearrangements,in which gene expression changes depending on the position of DNA sequencs in the genome.
The regulatory systems of prokayotes and eukaryotes differ from each other in many details.Because the project in the iGEM 's platforms are mostly prokayotes,we just focus on the prokayotes' gene regulation.Prokayotes are generally free-living unicellular organisms that grow and divice indefinitely as long as environmental conditions are suitable and the supply of nutrients is adequate.Their regulatory systems are often geared to provide the maximum growth rate in a particular environment .In contrast ,the cells in a developing muticellular organism modulate their growth rate as they undergo dramatic,coordinated differentiation in morphology and metabolism.In an adult animal,growth and division of most cell types has ceased,and each type of cell needs to maintain its identity through time.
Synthetic biology is a brand new field of biological research which combines science and engineering. Now more and more people are familiar with this promising and appealing field. Gene expression regulation can expand the regulating tools for synthetic biology. We can control protein biosynthesis through things like riboswitch. Different combinations of these regulating tools can bring different outcomes in protein expression levels. And they can be applied to real life to solve many difficult practical problems.
According to some of the championship projects in2011 igem,we find some are intresting and develpmental.Gene regulation can be called as the core for most of the projects.
For example, As BYU's first iGEM team, BYU team proposed constructing a molecular AND gate in E. coli. To detect their chosen inputs they investigated the OxyR promoter and a thermo-sensitive riboswitch. The OxyR protein activates transcription in the presence of hydrogen peroxide, a reactive oxygen speesscies (ROS). The riboswitch allows translation only above a specific temperature. Both inputs activate a Cre/Lox system to remove a terminator sequence and allow transcription of a molecular signal. And they think a similar system, in theory, could be used for early detection of colorectal cancer.
Another team is the SJTU-BioX-Shanghai, they designed a set of Codon-Switches that regulate target protein biosynthesis (translation).In their rare-codon switch, the translation of the protein can be finely turned up/down with the control of rare tRNA amount,aaRS that charges the rare tRNA and rare codons. Besides, their also made other two switches that can be turned on/off without background noise . One is to use stop codon as the controlling element, the stop-codon switch.The other is to use any codon but the original start codon to initiate translation, the initial-codon switch.
Various methods of regulation are provide.Followings are the typical ones.
Regulation with density: XMU gold medal,Advance to Championship
They have developed a series of devices which program a bacteria population to maintain at different cell densities. They have designed and characterized the genetic circuit to establish a bacterial ‘population-control’ device in E. coli based on the well-known quorum-sensing system from Vibrio fischeri, which autonomously regulates the density of an E. coli population. The cell density however is influenced by the expression levels of a killer gene (ccdB) in our device. As such, we have successfully controlled the expression levels of ccdB by using RBSes of different strength and mutated luxR promoters (lux pr). They are working on builting up a database for a series of mutation sites and RBSes corresponding to different steady-state cell densities. An artificial neural network will be built to model and predict the cell density of an E. coli population. This work can serve as a foundation for future advances involving fermentation industry and information processing.
Regulation with Light
WHU, Gold medal, Advance to Championship
Their project focuses on constructing colorful E.coli, which includes two parts. They plan to construct two systems consisting of several strains of E.coli: one produces different pigments due to the change of time, and the other produces different pigments with the change of position.
In the first part, the strain of E.coli works as an oscillator which can yield different kinds of pigment periodically with the help of a signal transformation system.
In the second part, They came up with the idea of “colorful E.film”. In hope to create a colorful film, They will construct three E.coli strains which can produce and secrete three primary colors respectively in the presence of the three primary lights.
UC Davis, gold medal
They set out to develop a quick, easy process for the expansion of basic parts into a part families. Our method employs a suped-up mutagenic PCR protocol that uses standard VF2 and VR primers and materials most iGEM teams already have on hand. They chose to prototype this process by creating a part family from the LacI promoter BBa_R0010, and to mutate GFP to visually assess our ability to create functional protein mutants.we have a functioning part family generation process and seven well-characterized LacI promoter mutants and eight GFP mutants (two of which have been lovingly named Orange-Mutated Green Fluorescent Protein or [OMGfp] 1 and 2) which await further characterization.
Regulation with temperature, UTP-Panama, Gold medal
To develop flexible and better sensors for environmental, agricultural and engineering applications are the aims of the UTP-Panama Team “SynBio Engineering Tool Kit”. In this way they work with Nitrate Biosensor (PyeaR - GFP composite) developed by Team BCCS-Bristol 2010, which expresses fluorescent signals upon nutrient detection, producing a high-resolution map of arable land. To achieve this goal they use the collateral effect of the AOX enzyme (Alternative oxidase) mainly designed to generate heat in response to a cold-shock, using the hybB promoter. This effect increases the bacteria growth at temperatures below 20°C. Finally we design a prototype device with a better cold shock promoter (CspA promoter) developed by UNAM-CINVESTAV Team in 2010, in order to give our E. coli a “Intelligent Coat", which means that not to only survive a cold-shock but to also still been able to keep up with his duties due to improve their expression mechanism at low temperature.
Regulation with Quorum-Sensing, THU,Gold medal
They dedicate in pursuing the goal of the construction of a biological oscillator, which they call 'ECHO', the abbreviation of 'the E. coli Homochronous Oscillator'. With two Escherichia coli populations expressing gene one after another, they give red and green fluorescent light alternately. E.coli populations communicate bi-directionally by a class of signaling molecules involves in bacteria quorum sensing, that is, N-Acyl homoserine lactones (AHL), to regulate the gene expression of each other. By engineering their gene circuits, two groups (name as CELL-A and CELL-B) will form a network, with B inducing A and A restricting B, thus able to realize oscillation. A mechanism is set up to change the rate of an AHL expression, allowing us to control the period and the phase of the oscillatory cycle.
As a translational regulatory tool, our device achieves more precise tuning of protein expression when compared with transcriptional tools. The controlling elements we use in this device are codons and tRNAs, the major participants of translation process. Thus manipulating these elements exert direct effects on protein biosynthesis. We just try to summarize something useful for more teams to refer to.
References
[1] ZHANG Zhen-tian, HUANG Guo-feng, ZHONG Liu-zhu. Genetic pollution and ecological and environmental safety[J]. Ecology and Environment 2005, 14(6): 987-989
[2] Dalton. R. Transgenic corn found growing in Mexico, Nature, 2011, 413:337
[3]QIAN Yingqian, WEI Wei, MA Keping. Thinking about the problems of biological safety. Impact of Science on Society, 2002(4):24-26
[4] WANG Xiu-mei. Gene Contamination, Biosafety and the Protection of International Environment——“The Protocol on Biosafety”and the New Development of International Environment Law[J]. Journal of Chang ′an University ( Social Science Edition) 2002, Vol. 4 No. 1
[5] LIU San-mao, CHEN Jin-xiang. Progress of transgene crop gene floating and it’ s gene pollution[J], JIANGXI COTTON 2005, Vol. 27, No. 6
[6] WEI Wei, Ma Keping. How Should We Face the Problems of Gene Flow and Gene Contamination[J], Review of China Agricul tural Science and Technology 2002, Vol. 4(4)
[7] LIANG Zhao. The current situation and problems of gene therapy[J]. Chinese Medical Ethics 2003(1):16-18
[8] WEI Xiaozhou. Genetic pollution---new century hardship[J]. Digest of Science and Technology 2004(7):10-11
[9] Daniel L. Hartl. Elizabeth W. Jones. Genetics. Jones and Bartlett Publishers. 2005
 "
"