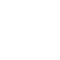Team:Tianjin/Project/Technology
From 2012.igem.org
(→Replace of RBS in Plasmid) |
(→MAGE) |
||
| Line 157: | Line 157: | ||
=MAGE= | =MAGE= | ||
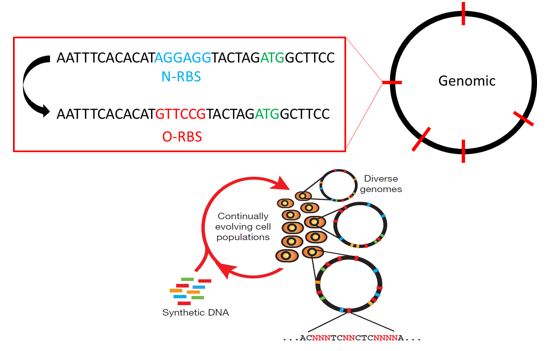
| - | We thought that now that we could introduce O-Lock cell, could we introduce O-Lock to the whole system. Theoretically speaking, we could make it come true because there is a technology called MAGE (Multiplex Automated Genomic Engineering) which comes from Harvard University. We could use this technology to replace all the N-RBS to O-RBS of the | + | We thought that now that we could introduce O-Lock into a cell, could we introduce O-Lock to the whole system. Theoretically speaking, we could make it come true because there is a technology called MAGE (Multiplex Automated Genomic Engineering) which comes from Harvard University. We could use this technology to replace all the N-RBS to O-RBS of the genome. Then we could regulate the metabolic network more freely and accurately by many AND gates which come from the O-Locks. |
[[file:TJU2012-Proj-LMR-fig-22.png|thumb|500px|center|'''Figure 6.''' The basic principle of MAGE (from TJU iGEM Team 2012)]] | [[file:TJU2012-Proj-LMR-fig-22.png|thumb|500px|center|'''Figure 6.''' The basic principle of MAGE (from TJU iGEM Team 2012)]] | ||
{{:Team:Tianjin/footer}} | {{:Team:Tianjin/footer}} | ||
Latest revision as of 01:18, 27 October 2012

Replace of RBS in Plasmid
Here, how did we introduce the O-Lock, we just use two primers to build pcr. Those two primers have about 20bp overhands on their 5' end, which contain the O-Locks or other mutations. Then we directly transform those PCR products to E. coli by electroporation-mediated method. Then those PCR productions could cyclize and form the complete plasmids.
Yeast Assembler
History
Yeast Assembler is based on in vivo homologous recombination in yeast. As for its high efficiency and ease to work with, in vivo homologous recombination in yeast has been widely used for gene cloning, plasmid construction and library creation. In the early of 2008, Zengyi Shao from University of lllinois at Urbana-Champaign, Urbana, used such a method to construct biochemical pathways. Such a method, for its high efficiency in assembling multiple genes, received great popularity since its appearance.
The Difficulty of Large Fragments Assembly
The general digestion connection will leave scar and will be limited by the specific cleavage sequences.
Long PCR fragment will suffer the decline of success rate and distortion, etc.
However, the construction of some large fragments cannot be avoided, so the development of a low-cost, simple operation, good fidelity, a little limiting factor large DNA fragment assembly method is particularly important.
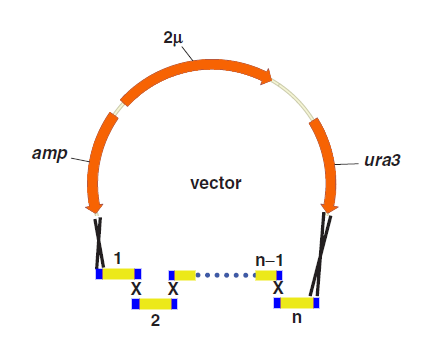
Principles
One step assembly into a vector.
When parts are transformed all parts into Yeast, homologous recombination occurs at the site “x”, and then all little parts are integrated into a vector.
Advantages and Disadvantages
Compared with other methods,the “Yeast assembler” are more efficient and useful for large gene assemble.
| Yeast Assembler | SLIC | Domino Method | |
|---|---|---|---|
| Advantages | High efficient, Fit for large gene assemble rapid | Fit for many little parts into overall | Fit for two large gene parts integration |
| Disadvatages | Process is complex | Success rate is low, The size of recombinant is relatively small(<8kb) | Require a great amount of time and labor. Process is sequential |
Completely Synthesizing the Genome of Mycoplasma Genitalium using Yeast Assembler
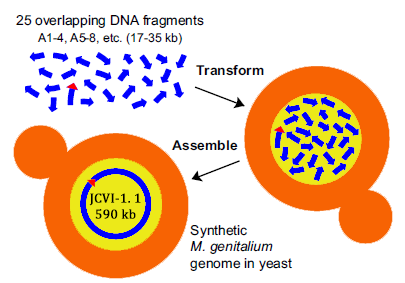
In 2008, Gibson from the J. Craig Venter Institute, published an article “one-step assembly in Yeast of 25 overlapping DNA fragments to form a complete synthetic Mycoplasma genitalium genome”. In the article, the author transformed 25 overlapping DNA fragments into Yeast, homologous recombination occurs, and then the whole genome is synthesized.
Construction of a synthetic M. genitalium genome in yeast. Yeast cells were transformed with 25 different overlapping A-series DNA segments (blue arrows; ~17 kb to35 kb each) composing the M. genitalium genome. To assemble these into a complete genome, a single yeast cell (tan) must take up at least one representative of the 25 different DNA fragments and incorporate them in the nucleus (yellow), where homologous recombination occurs. This assembled genome, called JCVI-1.1, is 590,011 bp, including the vector sequence (red triangle) shown internal to A86 – 89.
MAGE
We thought that now that we could introduce O-Lock into a cell, could we introduce O-Lock to the whole system. Theoretically speaking, we could make it come true because there is a technology called MAGE (Multiplex Automated Genomic Engineering) which comes from Harvard University. We could use this technology to replace all the N-RBS to O-RBS of the genome. Then we could regulate the metabolic network more freely and accurately by many AND gates which come from the O-Locks.
 "
"