Team:Tianjin/Project/OrthogonalSystem
From 2012.igem.org
Austinamens (Talk | contribs) (→References) |
Austinamens (Talk | contribs) (→References) |
||
| Line 174: | Line 174: | ||
=References= | =References= | ||
#http://en.wikipedia.org/wiki/16S_ribosomal_RNA | #http://en.wikipedia.org/wiki/16S_ribosomal_RNA | ||
| - | |||
#Lon M. Chubiz and Christopher V. Rao(2008). Computational design of orthogonal ribosomes, vol.36 no.12 | #Lon M. Chubiz and Christopher V. Rao(2008). Computational design of orthogonal ribosomes, vol.36 no.12 | ||
| - | |||
#Howard M Salis, Ethan A Mirsky & Christopher A Voigt(2009).Automated design of synthetic ribosome binding sites to control protein expression, doi:10.1038/nbt.1568 | #Howard M Salis, Ethan A Mirsky & Christopher A Voigt(2009).Automated design of synthetic ribosome binding sites to control protein expression, doi:10.1038/nbt.1568 | ||
| - | |||
#Wenlin An and Jason W. Chinl(2009).Synthesis of orthogonal transcription-translation networks,vol.106 no.21 | #Wenlin An and Jason W. Chinl(2009).Synthesis of orthogonal transcription-translation networks,vol.106 no.21 | ||
| - | |||
#George H.McArthur IV and Stephen S. Fong, Toward Engineering Synthetic Microbial Metabolism | #George H.McArthur IV and Stephen S. Fong, Toward Engineering Synthetic Microbial Metabolism | ||
| - | |||
#Kevin Clancy and Christopher A Voigt, Programming cells: towards an automated ‘Genetic Compiler’ | #Kevin Clancy and Christopher A Voigt, Programming cells: towards an automated ‘Genetic Compiler’ | ||
| - | |||
#WANG Haisheng, ZHANG Xiaoxia, et al., Recent research progress of bacterial violacein | #WANG Haisheng, ZHANG Xiaoxia, et al., Recent research progress of bacterial violacein | ||
| - | |||
#Carotenoid Crystal Formation in Arabidopsis and Carrot Roots Caused by Increased Phytoene Synthase Protein | #Carotenoid Crystal Formation in Arabidopsis and Carrot Roots Caused by Increased Phytoene Synthase Protein | ||
Levels | Levels | ||
| - | |||
#Guoyin Kai, Pan Liao, Tong Zhang, Wei Zhou, Jing Wang, Hui Xu, Yuanyun Liu, and Lin Zhang, Characterization, Expression Profiling, and Functional Identification of a Gene Encoding Geranylgeranyl Diphosphate Synthase from Salvia miltiorrhiza. | #Guoyin Kai, Pan Liao, Tong Zhang, Wei Zhou, Jing Wang, Hui Xu, Yuanyun Liu, and Lin Zhang, Characterization, Expression Profiling, and Functional Identification of a Gene Encoding Geranylgeranyl Diphosphate Synthase from Salvia miltiorrhiza. | ||
| - | |||
#crtB Masaki Fujisawa,Mio Watanabe,Song-Kang Choi,Maki Teramoto,Kanji Ohyama,and Norihiko Misawa, Enrichment of Carotenoids in Flaxseed (Linum usitatissimum) by Metabolic Engineering with Introduction of Bacterial Phytoene Synthase Gene | #crtB Masaki Fujisawa,Mio Watanabe,Song-Kang Choi,Maki Teramoto,Kanji Ohyama,and Norihiko Misawa, Enrichment of Carotenoids in Flaxseed (Linum usitatissimum) by Metabolic Engineering with Introduction of Bacterial Phytoene Synthase Gene | ||
| - | |||
#William R. Farmer and James C. Liao, Improving lycopene production in Escherichia coli by engineering metabolic control. | #William R. Farmer and James C. Liao, Improving lycopene production in Escherichia coli by engineering metabolic control. | ||
{{:Team:Tianjin/footer}} | {{:Team:Tianjin/footer}} | ||
Revision as of 03:48, 27 September 2012

The Orthogonal System of Regulating Translation: the AegiSafe™ O-Key
The synthesis of protein relies on the transcription-translation network. In transcription, mRNA is synthesized through complementary base pairing by RNA polymerase from the DNA template, and is followed by translation. Translation is the third stage of protein biosynthesis. In translation, mRNA produced by transcription is decoded by the ribosome to produce a specific amino acid chain, or polypeptide, that will later fold into an active protein. Therefore, we can say that translation is one of most important of activities in a cell.Due to its importance of protein synthesis, there are intricate and delicate regulation systems. To regulate transcription, cells alter the gene expression levels. This is called transcriptional regulation. Many means of transcriptional regulation, such as various mechanisms as specificity factors, activators, etc., are presented in a cell. There are also inducible and repressible systems, and transcription factor that can determine the initiation rate of transcription.
While a number of genetic tools exist for regulating transcription in cells, far fewer tools exist for translation. Of the tools available in bacteria, the most popular are riboregulators, both cis- and trans-activating, and orthogonal ribosomes (o-ribosomes). In terms of reprograming translation, o-ribosomes are especially powerful as they enable one to partially decouple translation from the native protein synthesis machinery. In particular, o-ribosomes can translate genes with altered Shine-Dalgarno (SD) sequences not recognized by host ribosomes. Therefore, o-ribosomes can be used to explore gene expression dynamics as they potentially provide a method for tuning translation rates. Furthermore, o-ribosomes may have application in synthetic biology as they introduce new functionality within cells.In this year's project, we focus on modifying the Sine-Dalgarno (SD) sequence of the mRNA and the anti-Shine-Dalgarno (ASD) sequence of the 16S rRNA of the ribosome to construct an orthogonal translation system called the AegiSafe™ O-Key. Aegis was originally the shield that was associated with Zeus and Athena, which offers protection. AegiSafe™ means our orthogonal system secure the environment from potential biosafety issue. O-Key stands for orthogonal system that includes orthogonal ribosome (o-ribosome, O-Key) and orthogonal RBS (o-RBS, O-Lock), which indicates that our system can not only shut down contamination, but also using the orthogonal system to selectively open our translation pathway like a key to a lcok. This is a truly amazing system for two independent translation systems co-existing harmoniously and working effectively in one single cell. In the following sections, we will elaborate on the AegiSafe™ O-Key about the orthogonal system and its application.
The Overview of Orthogonal Ribosome (O-Key)
The Canonical Ribosome
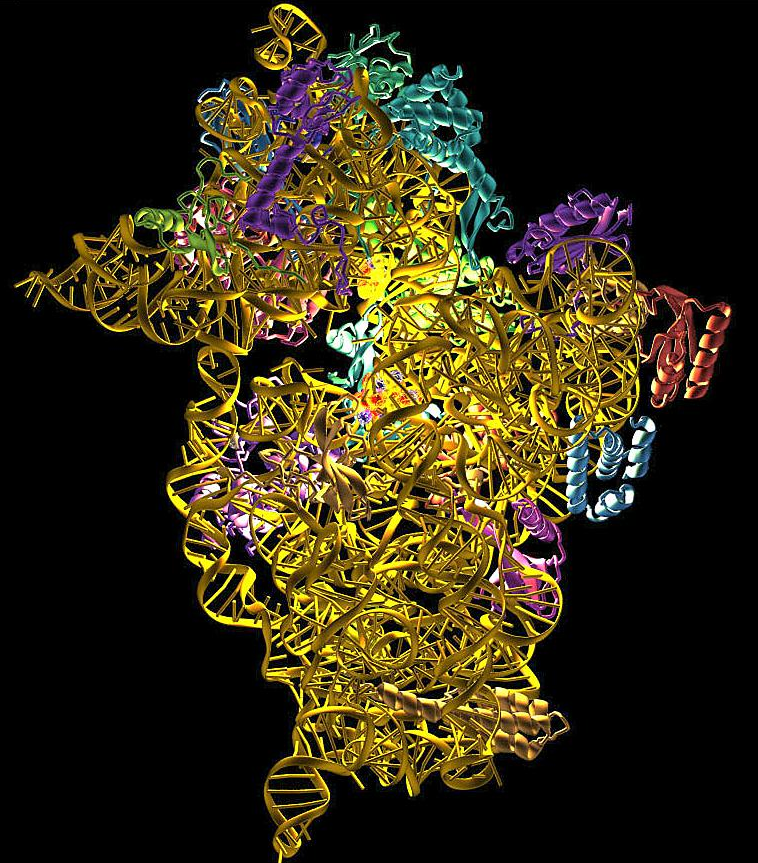
Ribosome is the protein factory of a cell, an organelle without membrane. Its sophisticated structure guarantees the accurate information flow from mRNA to protein. Ribosomes consist of two subunits that fit together and work as one to translate the mRNA into a polypeptide chain during protein synthesis.
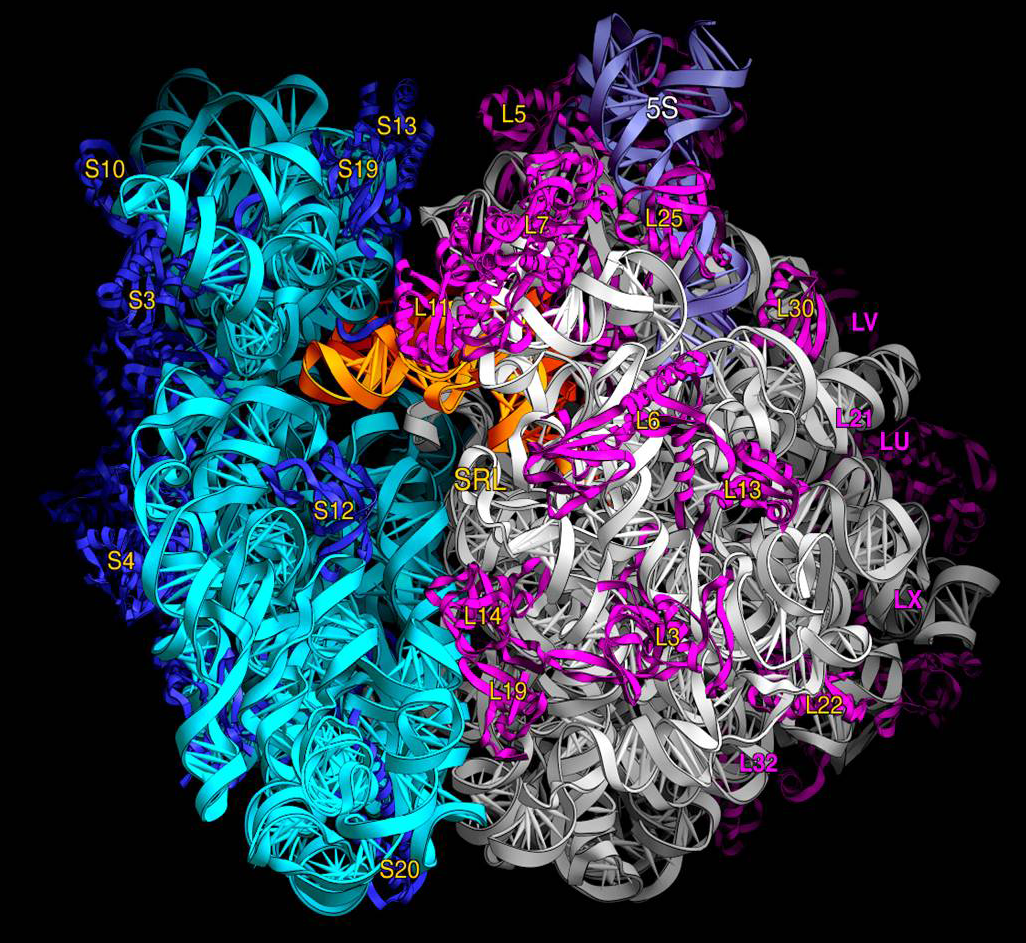
Prokaryotes have 70S ribosomes, each consisting of a small (30S) and a large (50S) subunit. The smaller subunit binds to the mRNA pattern, while the larger subunit binds to the tRNA and the amino acids. Their small subunit has a 16S RNA subunit (consisting of 1540 nucleotides) bound to 21 proteins. The large subunit is composed of a 5S RNA subunit (120 nucleotides), a 23S RNA subunit (2900 nucleotides) and 31 proteins. Crystallographic work has shown that there are no ribosomal proteins close to the reaction site for polypeptide synthesis. This suggests that the protein components of ribosomes act as a scaffold that may enhance the ability of rRNA to synthesize protein rather than directly participating in catalysis, while the rRNA reacts with the mRNA and catalyze the synthesis.
Multiple sequences of 16S rRNA can exist within a single bacterium.
It has several functions:
- Like the large (23S) ribosomal RNA, it has a structural role, acting as a scaffold defining the positions of the rebosomal proteins.
- The 3' end contains the anti-Shine-Dalgarno sequence, which binds upstream to the AUG start codon on the mRNA. The 3'-end of 16S RNA binds to the proteins S1 and S21 known to be involved in initiation of protein synthesis
- Interacts with 23S, aiding in the binding of the two ribosomal subunits (50S+30S)
- Stabilizes correct codon-anticodon pairing in the A site, via a hydrogen bond formation between the N1 atom of Adenine (see image of Purine chemical structure) residues 1492 and 1493 and the 2'OH group of the mRNA backbone.
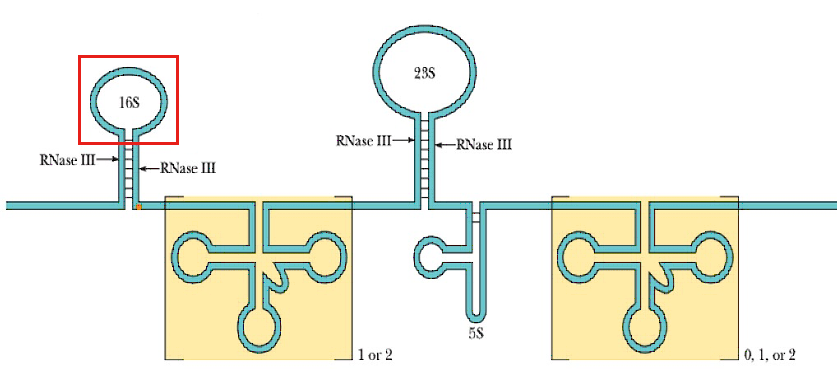
In bacterial cells, ribosomes are synthesized in the cytoplasm through the transcription of multiple ribosome gene operons.
The rRNAs that make up the ribosome are directly translated from the rRNA operon rrnB. The three E. coli rRNA molecules – 23S, 16S, and 5S – are derived from a single 30S rRNA precursor transcript that also includes several tRNAs.
The Orthogonal Ribosome
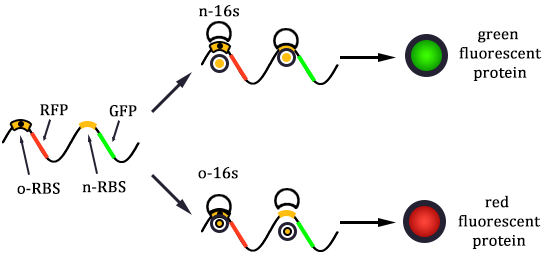
The previous section described the structure of a canonical ribosome. As for the orthogonal ribosome (O-Key), the primary structure remains the same. The only difference lies in the ASD sequence close the 3' of the 16S rRNA. This altered sequence will not recognize the canonical SD sequence, thus pausing translation, just like a key cannot open a mismatched lock. The O-Key can only pair with its corresponding RBS, i.e. O-Lock. The O-Key (orthogonal) system serves as a powerful tool in regulating inner cellular metabolism. We will elaborate on its application in the following chapter.
Gibson Free Energy in the O-Key System
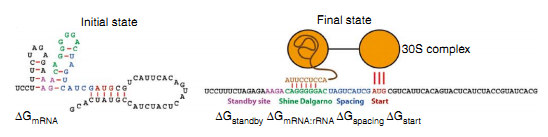
The strength of the interaction between SD and ASD sequence is thought to influence translational efficiency as mutations in either the SD or ASD sequence that weaken the interaction reduce the amount of protein made. The mechanism of protein expression is primarily determined by the delta Gibbs free energy of the combination of SD sequence on ribosome and the ASD sequence on RBS of the mRNA. Therefore, it is necessary to investigate the property of ΔG.
ΔGtot=ΔGmRNA:rRNA+ΔGstart+ΔGspacing-ΔGstandby-ΔGmRNA
ΔGmRNA:rRNA is the energy released when the last nine nucleotides(nt) of the E. coli 16S rRNA (3′-AUUCCUCCA-5′) hybridizes and co-folds to the mRNA sub-sequence (ΔGmRNA:rRNA<0). Intramolecular folding within the mRNA is allowed. All possible hybridizations between the mRNA and 16S rRNA are considered to find the highest affinity 16S rRNA binding site. The binding site minimizes the sum of the hybridization free energy ΔGmRNA:rRNA and the penalty for nonoptimal spacing, ΔGspacing. Thus, the algorithm can identify the 16S rRNA binding site regardless of its similarity to the consensus Shine-Dalgarno sequence.
- ΔGstart is the energy released when the start codon hybridizes to the initiating tRNA anticodon loop (3'-UAC-5').
- ΔGspacing is the free energy penalty caused by a nonoptimal physical distance between the 16S rRNA binding site and the start codon (ΔGspacing>0). When this distance is increased or decreased from an optimum of 5 nt (or ~17A), the 30S complex becomes distorted, resulting in a decreased translation initiation rate.
- ΔGmRNA is the work required to unfold the mRNA sub-sequence when it folds to its most stable secondary structure, called the minimum free energy structure (ΔGmRNA<0).
- ΔGstandby is the work required to unfold any secondary structures sequestering the standby site (ΔGstandby<0) after the 30S complex assembly. We define the standby site as the four nucleotides upstream of the 16S rRNA binding site, which is its location in a previously studied mRNA.
To calculate ΔGmRNA:rRNA, ΔGstart, ΔGmRNA and ΔGstandby, we use the NUPACK suite of algorithms with the Mfold 3.0 RNA energy parameters. These free energy calculations do not have any additional fitting or training parameters and explicitly depend on the mRNA sequence. In addition, the free energy terms are not orthogonal; changing a single nucleotide can potentially affect multiple energy terms. The relationship between the spacing and the ΔGspacing was empirically determined by measuring the protein expression level driven by synthetic RBSs of varying spacing and fitting a quantitative model to this data.
Orthogonality Verification Experiment
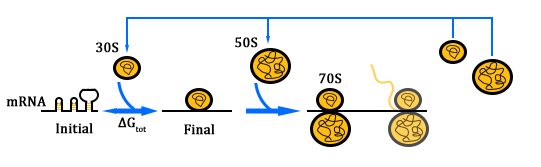
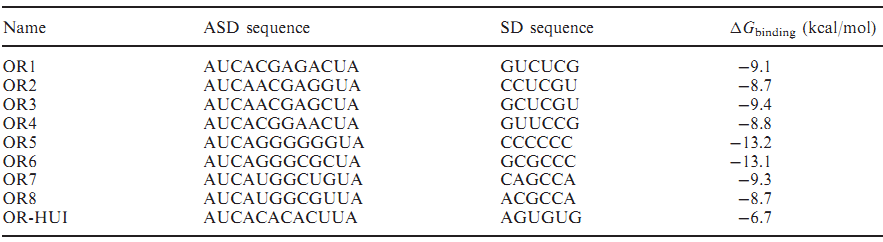
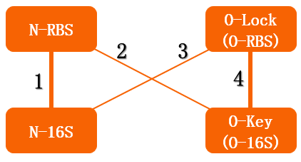
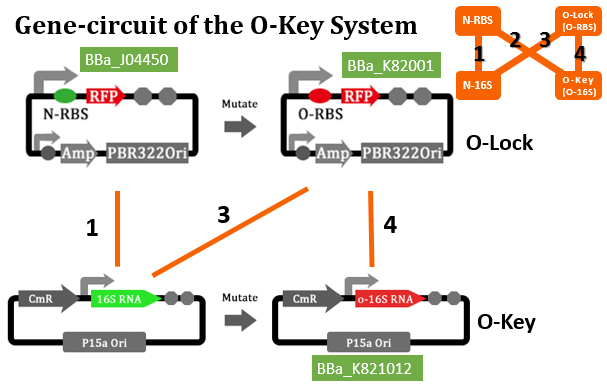
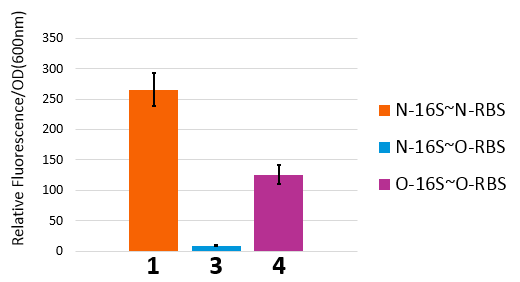
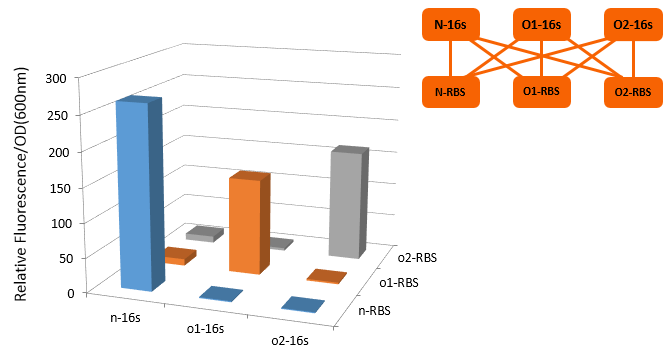
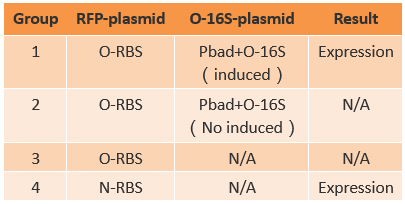
If orthogonal RBS is safe, why don't we change all the canonical 16s into orthogonal 16s? The answer is no. That is because if we change all the base 16s, the other housekeeping genes will stop expressing. This will produce orthogonal rivalry, leading to bacterial death. So we are bound to face competition between canonical and orthogonal 16s. Then we design the following experiment to research this problem. We designed three operons to verify the orthogonality of our system. First, our first experiment is based on an assumption that the fluorescent light intensity can represent the amount of the expression of fluorescent protein. The relevant literature indicates that the fluorescent light intensity represents the amount of the expression of fluorescent protein and the assumption can be right if the experimental precision is not sensitive.
We constructed two sets of plasmid co-transformed into E. coli to achieve the purpose. One set is induced by the promoter then transcripts the O-key's vector, which is equivalent to the key. The other set is O-RBS/RFP+RBS/GFP, it is constructed to compare the orthogonality between the O-RBS and N-RBS by GFP and RFP.
From this figure, we can see that there are four competition relationships in the orthogonal cell system, the ideal state is the 1th and 2th path exists while the 3th and 4th path weaken. That means our system has good orthogonality. We did multiple sets of pre-experiments of different O-16s then decided to use this set.
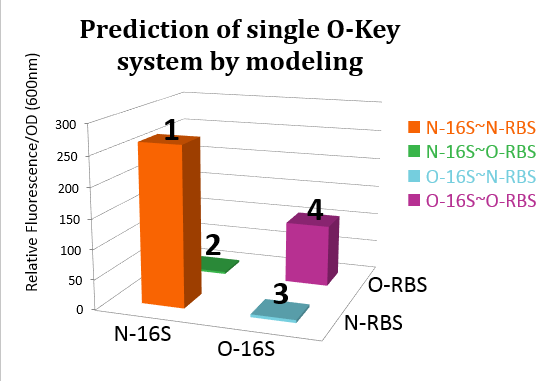
Modeling
In order to design the orthogonality and to predict the protein expression level, we have established the protein expression model which has strong relationship with the wet lab. The model is based on detailed study of the effects of changing Gibbs free energy on the translation process.
In the process of translation, the initiation rate is the rate determine step. The protein expression amount is consequently direct proportional to the initial translation speed. Furthermore, the initial translation speed is strongly related to the delta Gibbs free energy, which can be calculated out through results of many newly published papers and various commercialized software such as the Vienna RNA Package, RBS-Calculator, and Nucleic Acid Package. In our model, we calculated the delta Gibbs energy. Ideal results can be obtained from the model. The orthogonality predicted by our model before the wet lab is similar to our prediction. When combined with some wet lab result, we can finally predict the spectroscope curve of RFP and GFP under three designed experimental state. For more details, see our Model Part.
Future Work
Previous experiments and modeling has described that the orthogonality of our expression system is very tight, but we are still not satisfied with the current state, then we design and do the following experiment. The following experiment includes:
- We are using λRed technology to integrate the O-16s biobrick into the genome for constructing the orthogonal cells, so as to solve the revertants as well as stability problems caused by plasmid instability.
- We are trying to build other groups of different orthogonal (O-16s-RBS) system, so as to achieve the different orthogonality switch effect, and attempt to make them corporate.
- We also consult with some biological gene enterprises to make our results put into production in the life full of modern technology, such as genetic pollution control, gene encryption and metabolic regulation, etc.
References
- http://en.wikipedia.org/wiki/16S_ribosomal_RNA
- Lon M. Chubiz and Christopher V. Rao(2008). Computational design of orthogonal ribosomes, vol.36 no.12
- Howard M Salis, Ethan A Mirsky & Christopher A Voigt(2009).Automated design of synthetic ribosome binding sites to control protein expression, doi:10.1038/nbt.1568
- Wenlin An and Jason W. Chinl(2009).Synthesis of orthogonal transcription-translation networks,vol.106 no.21
- George H.McArthur IV and Stephen S. Fong, Toward Engineering Synthetic Microbial Metabolism
- Kevin Clancy and Christopher A Voigt, Programming cells: towards an automated ‘Genetic Compiler’
- WANG Haisheng, ZHANG Xiaoxia, et al., Recent research progress of bacterial violacein
- Carotenoid Crystal Formation in Arabidopsis and Carrot Roots Caused by Increased Phytoene Synthase Protein
Levels
- Guoyin Kai, Pan Liao, Tong Zhang, Wei Zhou, Jing Wang, Hui Xu, Yuanyun Liu, and Lin Zhang, Characterization, Expression Profiling, and Functional Identification of a Gene Encoding Geranylgeranyl Diphosphate Synthase from Salvia miltiorrhiza.
- crtB Masaki Fujisawa,Mio Watanabe,Song-Kang Choi,Maki Teramoto,Kanji Ohyama,and Norihiko Misawa, Enrichment of Carotenoids in Flaxseed (Linum usitatissimum) by Metabolic Engineering with Introduction of Bacterial Phytoene Synthase Gene
- William R. Farmer and James C. Liao, Improving lycopene production in Escherichia coli by engineering metabolic control.
 "
"