Team:TU-Delft/HP/Study
From 2012.igem.org
| (5 intermediate revisions not shown) | |||
| Line 32: | Line 32: | ||
<p>These are six main diagnosis tools for TB. The problem is that there is not one of them that is very reliable, fast and also suitable for the frontline at the same time. Not suitable for the frontline because it needs extensive facilities, doesn't give a quick result and/or is expensive.</p> | <p>These are six main diagnosis tools for TB. The problem is that there is not one of them that is very reliable, fast and also suitable for the frontline at the same time. Not suitable for the frontline because it needs extensive facilities, doesn't give a quick result and/or is expensive.</p> | ||
<body><table> | <body><table> | ||
| - | <td width=" | + | <td width="80%"> |
<ul> | <ul> | ||
<li>Tuberculin Skin Test (TST) | <li>Tuberculin Skin Test (TST) | ||
| Line 43: | Line 43: | ||
</td> | </td> | ||
<td> | <td> | ||
| - | <a href="http://upload.wikimedia.org/wikipedia/commons/a/a5/%22Guard_Against_Tuberculosis%22_-_NARA_-_514425.jpg"><img src="http://upload.wikimedia.org/wikipedia/commons/a/a5/%22Guard_Against_Tuberculosis%22_-_NARA_-_514425.jpg" width="150"/><br><h6> | + | <a href="http://upload.wikimedia.org/wikipedia/commons/a/a5/%22Guard_Against_Tuberculosis%22_-_NARA_-_514425.jpg"><img src="http://upload.wikimedia.org/wikipedia/commons/a/a5/%22Guard_Against_Tuberculosis%22_-_NARA_-_514425.jpg" width="150"/><br><h6>Source: wikimedia commons</h6></a> |
</td> | </td> | ||
</table> | </table> | ||
Latest revision as of 02:06, 27 October 2012


Implementation Study
For our Human Practice we did a study in to what extent our proof of principle when translated into a product, could make a difference. To answer this we evaluate the most important parts of these questions below.
What is Tuberculosis?
Tuberculosis is a bacterial infection. TB cells usually attack the lungs, but can also attack other parts of the body, such as the brain, spine, or kidneys. TB bacteria can live in the body without making a person sick. This is called latent TB infection. People with latent TB infection do not feel sick, do not have TB symptoms, and cannot spread TB bacteria to others. People with latent TB infection go on to develop TB disease.
TB becomes even a larger problem because of HIV: 60.1% of TB patients tested for HIV were HIV-positive. HIV-patients with TB have to be cured even faster because of their immune system is weakened.
In 2010, 8.8 million people were infected with TB and 1.4 million died from it. Over 95% of TB deaths occur in low -and middle- income countries.

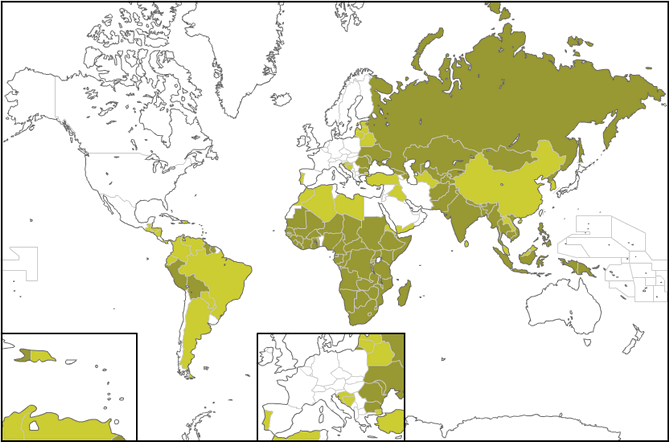
At the map below you can see the countries suffering from Tuberculosis. According to www.vaccinatiesopreis.nl the dark green refers to many cases of TB, the light green to less and the white to a few cases.
The problem
The problem of tuberculosis lies mainly in the less developed countries. The frontline are the remote areas where the people have no or limited access to hospitals with sufficient facilities. Standard TB diagnostic tools are either expensive and need to be used in a lab setting or are not very reliable, all these reasons are posing major barriers for diagnosing.
Six Ways to Diagnose Tuberculosis
These are six main diagnosis tools for TB. The problem is that there is not one of them that is very reliable, fast and also suitable for the frontline at the same time. Not suitable for the frontline because it needs extensive facilities, doesn't give a quick result and/or is expensive.
|
 Source: wikimedia commons |
Our ideal Solution
The intended use of the device is in a diagnosis area in a controlled environment like a medical facility or even town hall. Further the device should be easy to operate, have low costs, and should give quickly a reliable result.
What would a test based on our principle contribute?
Reach
Yeast cells can be kept in dried form, which makes them very well transportable and easy to store. Therefore remote areas can be easily reached. In cool areas (4 °C) dry yeast can be contained for two years. The environment to grow yeast in dry warm countries is far from optimal, but encapsulating the yeast growth in a small container can facilitate growth. A container with temperature indicator can serve as guideline to see where it has to be placed during the measurement. It should also have a filter which exchanges gasses coming from the yeast and the TB bacteria and the environment, holding the organisms in the container. Ideally the area between the growing media and the air is large, without drying out.Affinity of receptor Gpr109A towards methyl nicotinate
Human Gpr109A expressed in HEK293 cells have affinities towards nicotinic acid going down to 204 +- 67 nM [1] concentrations, the rat receptor 400 nM [2] concentrations (EC50 values). For yeast an EC50 concentration of 904 +- 28 nM2 is measured when human Gpr109A receptors are expressed together with the Gα chimeric subunit. Although a lot of derivatives are tested, information about affinities with methyl nicotinate are not found in literature. Structural simulations indicate that the binding energy (10 ns simulation) in the binding niche decreases approximately 50% in comparison with Niacin, the original ligand in the original receptor. This would result in lower affinity and this is why mutagenic studies on these receptors have been performed. The results of this can be found in the structural model page.Concentrations methyl nicotinate found in TB patients respiratory tract
There are two general ways to find infection of TB cells in the respiratory tract. Either by analysis of the sputum or the breath of a tuberculosis patient. The analysis of breath can facilitate the diagnosis, as shown by the Syhre et al. of the university of Otago. The concentrations coming of tuberculosis patients is however very low, towards the femtomolar range. When the methyl nicotinate concentration in the breath is compared with the affinity of the reported receptors these concentrations are expected to be too low. The method used to let the species Rattus norvegicus identify TB cells is through analysis of plated sputum of TB patients [3]. This way the speed of analysis and cost is improved in comparison with the general analysis through microscopy of acid-alcohol fast stained sputum samples [4]. Plating of the sputum gives an exponential increase in tuberculosis cells present in this sputum, thus generating an exponential increase of methyl nicotinate over time [5]. Thereby adding yeast media and dry yeast, yeast will glow up when the tuberculosis molecule is present. The sputum concentration of tuberculosis is 3 nmol [6].Whether this, in combination with the near presence of the TB source activates the receptor affinity is achievable is yet to be tested.Measurement techniques
| The Snifferomyces cells ideally generate light with minimal methyl nicotinate concentrations. The measurement can be measured when excited with a monochromatic light. A LED can serve as source for minimal spectral source, shown in the design of the Sniffer-o-meter. A light sensitive resistor can translate the smallest voltage difference into a numerical indication by an ADC chip. Signal amplification in the yeast and device and noise reduction in the device are to be characterized. Receptor activity is generally measured using the FLIPR technique [7]. Using a similar light sensitive Ca2+ indicator system the yeast cells can be monitored on opening a co-expressed ion channel, activated by a receptor. A drawback here would be the interfering calcium transport of both TB and yeast cells. |  |
Evaluating of test result
We tested the Sniffer-o-meter and it is concluded that the device is capable of detecting the glowing GFP. So by reading of the Voltage from the voltmeter and compare the results to a reference, one can concluded weather a patient is infected or not. For a more detailed version, please go to the Sniffer-o-meter page on our wiki.Costs
Compared to the other diagnosing method for TB, the yeast cells used in the Sniffer-o-meter are the most cheaply replaceable parts. Furthermore the expenses of the device itself would sum up to a total of approximately 47 euro with integrated Voltmeter readout, see the compound sheet on the Sniffer-o-meter page. By comparing the cost of the prototype of the Sniffer-o-meter and considering the low yeast costs with the other diagnosing methods cost per test, one can concluded that our method will be the cheapest.Reliability
Before the device would be on the market for actual use, more research, testing and evaluation are needed to give responsible scientific and ethical answer on the reliability.Waiting time
Hopefully the state of the GPCR cascade can be optimized before it is dried, leaving the enzymatic state intact. Yeast cells can serve as a fast (~4 hours) one time use device since it is a generally cheap method. In case of an inducible method, the protein concentrations can be altered one hour before the measurement. Before this the yeast has to be inoculated. Therefore waiting time for the result will be approximately four hours.Safety
While making the device, the team also thought of several safety questions. One of the main questions would be: How will the organisms act outside of the device? Since we designed the organisms ourselves, we know that the yeast cells would not be able to survive on a different medium, in this case would be outside of the Sniffer-o-meter. The organisms are kept in a special container, and on the device itself is a warning placed for the operator, which states that this device should not be demolished. With all this in mind we could also say that the environment would not be negatively affected by the exposure of the modified organisms. One can concluded that it would not influence the ecology of the Earth, since we know that the yeast is not beneficial designed for survival.Conclusion
The advantages of the Sniffer-o-meter:
• Non-invasive: the device only needs a sample of saliva of the patient.
• Fast: to obtain the final results of the test it will take approximately 4 hours.
• Cheap: compared to all the other diagnosing methods, the Sniffer-o-meter would be the cheapest to use.
• Accessible: the Sniffer-o-meter is a small and portable device. This means that this way of diagnosing can take place in environments up to +- 30 Celsius when cooled supply is possible.
• Operational: with the clear operational instructions the device would be easy to operate. And it would not be necessary to have professionally trained medical people.
• Safety: it is a safe device to use since the modified organisms do not have a chance to survive outside the device.
We strongly believe that our project is well worth to be further developed. We believe it can make a change in how diagnostics in remote areas take place. But also for areas with facilities, it can be a cheap alternative for the diagnosing of other diseases that can be detected by specific compounds.
.
Example Mozambique; lack of facilities
Mozambique ranks the 19th among the 22 Tuberculosis High Burden Countries 2011 according to the WHO.One of our team members, Isabelle, traveled to Mozambique in July 2011. The lack of the diagnostic capacity is very clear at the hospital of Ilha de Mocambique. Ilha de Mocambique inhabits 14.000 people and they are relying on the hospital there. This picture of the Hospital is taken in July 2011. In contrary to what Wikipedia states, part of this building is still being used as a hospital.

References
[1] Ilona Mandrica et al. Evidence for constitutive dimerization of niacin receptor subtypes (2010) Biochem. and Biophys. Research Comm. Vol. 395 pp. 281–287
[2] Alan Wise et al. Molecular Identification of High and Low Affinity Receptors for Nicotinic Acid (2003) The Journal of Biol. Chem. Vol. 278, No.11, pp 9869-9874
[3] Georgies F. Mgode et al. Diagnosis of Tuberculosis by Trained African Giant Pouched Rats and Confounding Impact of Pathogens and Microflora of the Respiratory Tract (2012) J. Clin. Microbiol. 50(2):27
[4] Mona Syhre et al. The scent of Mycobacterium tuberculosis – Part II breath (2009) Tuberculosis 89 263–266
[5] Mona Syhre and Stephen T. Chambers The scent of Mycobacterium tuberculosis (2008) Tuberculosis 88, 317–323
[6] Monay Syhre the scent of Mycobacterium tuberculosis – Part II breath (2009) Tuberculosis 89 263–266
[7] Irina Vetter Development and Optimization of FLIPR High Throughput Calcium Assays for Ion Channels and GPCRs (2012) Advances in Experimental Medicine and Biology Volume 740, 45-82

 "
"