Biosynthesis - Fatty Acid
Background
Fatty acid derivatives are to emerge as prominent fuels in the marketplace. Their high energy density and low water solubility make them promising alternatives to fossil fuel as transportation fuels. Biosynthesis, among all the biofuels production methods, is the one most sustainable, environmental friendly and less controversial.
Fatty acid biosynthesis in E.coli is catalyzed by a series of nine enzymes and the release of free fatty acids is catalyzed by a thioesterase via hydrolysis of acyl-ACP species. We are the first group in iGEM competition to recruit this enzyme system to facilitate the biosynthesis of fatty acid, especially C18 fatty acid.
We put Membrane Accelerator system into practice context to justify its versatility and its efficiency.
Biosynthetic Pathway
Fatty acid biosynthesis in E.coli is carried out by a nine-component enzyme system , FabA, FabB, FabD, FabF, FabG, FabH, FabI, FabZ, and ACP. They orderly cooperate to convert one equivalent of acetyl-CoA and 6-8 equivalents of malonyl-CoA into C14-C18 acyl-ACP species. The cytoplasmic mutant of the periplasmic thioesterase is capable of releasing free fatty acids preventing the fatty acyl yields from being directly harnessed for phospholipid biosynthesis.
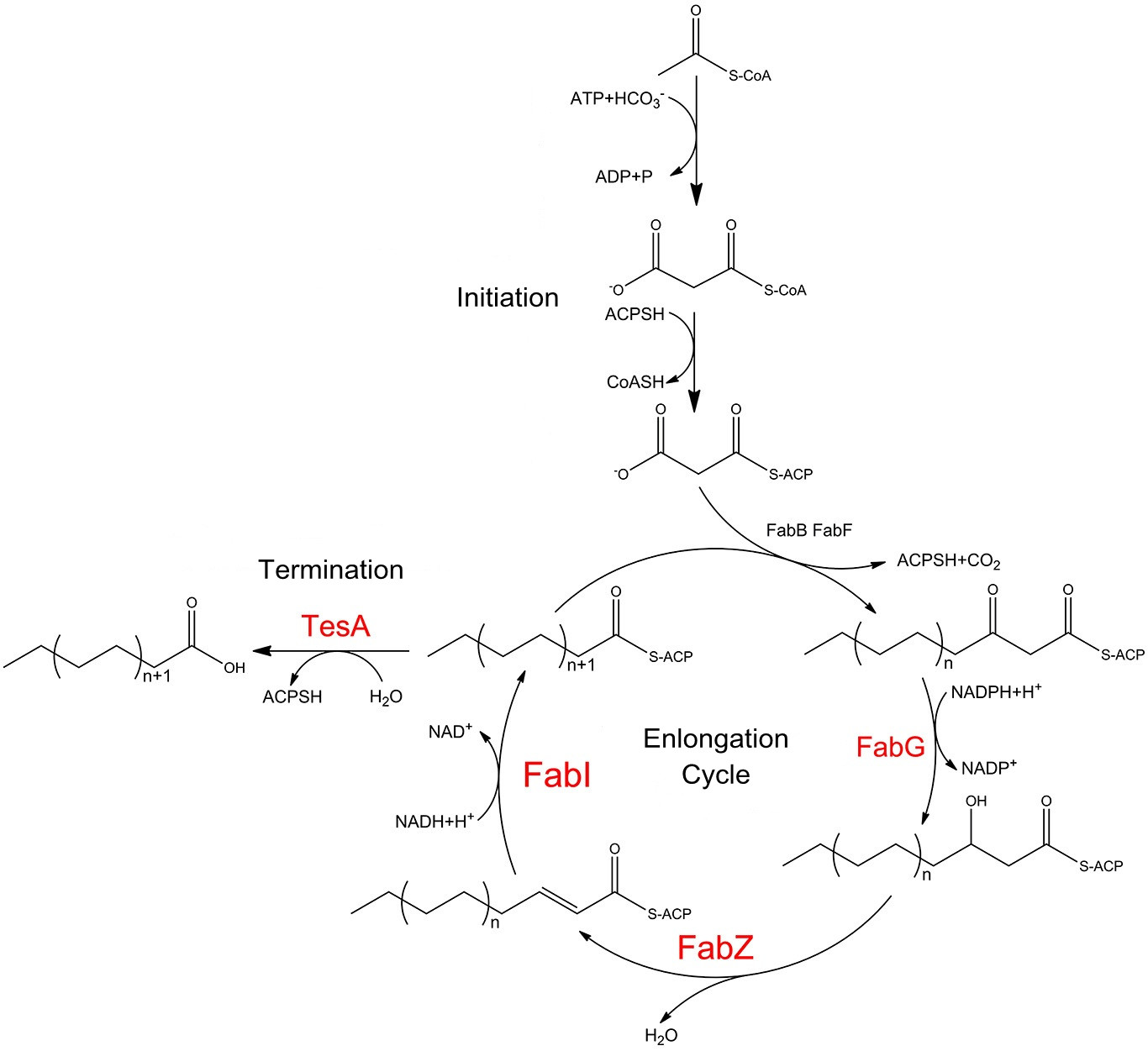 Fatty acid biosynthesis pathways in E.coli, showing the role of FabG, FabZ, FabI and TesA. Catalytic cycle of the E. coli fatty acid synthesis is initiated when holo-ACP, NADPH and NADH, acetyl-CoA and malonyl-CoA undergo condensation and subsequent reduction to form butyryl-ACP. These reactions are catalyzed by the malonyl-CoA:ACP transacylase FabD, the ketosynthase FabH, the NADPH-dependent ketoreductase FabG, either the dual-function dehydratase/isomerase FabA or the monofunctional dehydratase FabZ, and the NADH-dependent enoyl reductase FabI.
Then the butyryl-ACP is extended via 5-7 rounds of analogous reactions to produce a C14 to C18-ACP either fully saturated or monounsaturated. These extension cycles are catalyzed by either the ketosynthase FabB or FabF in collaboration with FabD, FabG, FabZ, and FabI.
Finally, the full-length fatty acid is released from the corresponding fatty acyl-ACP via hydrolysis by C16-specific thioesterase,TesA.
Design of Experiment
Mechanism beneath our idea suggests the Membrane Magic project enjoys two fundamental privileges: the Priority to Exportation and the Refinement of Interaction.
The Priority to Exportation
The fatty acid synthesis is terminated by the hydrolysis of fatty acyl-ACP and the release of free fatty acids into cytoplasm. We suppose TesA anchored on membrane could effectively increase the concentration of fatty acids near membrane, which in turn, facilitates the transmembrane transportation of fatty acids. Higher level of fatty acids in the culture medium make it easier to obtain and purify product and more suitable for Industrialized production. On the other hand, TesA removes fatty acyl-ACP from the reaction and thus, according to Le Chatelie principle, shifts the chemical equilibrium to accelerate and to accumulate more fatty acids.
The Refinement of Interaction
Enzymes fused with membrane anchors will be directed to the membrane as expected soon after their translation. Thus, the distribution of enzymes is restricted to the 2D membrane rather than diffusing randomly throughout the 3D cytoplasm. Due to spatial restriction, we expect the receptor-ligand interaction that we employ to form enzymes cluster occurs at a higher frequency comparing to scafolds in other forms. Moreover, the interaction could be stabilized by phospholipid around transmembrane domain. We believe a tandem of enzymes involved in sequential reactions could be established on the membrane swiftly and orderly.
To identify and to evaluate the advantages of the system, two sets of controlled experiments were designed and conducted.
Four enzymes are selected based on previous study and fusion proteins are constructed to form protein complex on the cell membrane. We express thioesterase, TesA and full complement of reductive enzymes, FabG, FabZ and FabI because moderate overexpression of TesA in gave rise to elevated fatty acid productivity and reductive enzymes could lead to 50% increase in fatty acid turnover.
TesA are fused with transmembrane protein No.1 as stated in the Construction and FabG, FabZ and FabI with No.2 to No.4 respectively, align with corresponding reactions occurring in sequence.
//上图
- WT stands for E.coli transformed with corresponding plasmid(s) without exogenous gene.
- F-protein here indicates enzyme diffusing randomly throughout the cytoplasm without modification.
- M-protein means enzyme fused with engineered transmembrane domine and localized to E.coli membrane where enzymes aggregate and cooperate.
Result and Discussion
Excitingly, the fatty acid biosynthesis was accelerated sharply by gathering downstream enzymes through interacting protein domains. The results showed that the production of Fatty acid was enhanced significantly by more than 20 fold through recruiting our membrane accelerator system, indicating a promising application prospect in biofuel production.
1. WT, F-TesA and M-TesA
20 hours after induction, E.coli expressing membrane anchored TesA experienced a 50% increase in fatty acids content of both supernatant and sedimentation, compared with E.coli expressing free TesA. Fatty acids yielded from sedimentation went up to 5.02mg/(L·OD) and that from supernatant 0.71mg/(L·OD).
We also witnessed a slight decrease in E.coli expressing free TesA compared with the wildtype, which testifies the statement in a previous study that high levels of TesA inhibits fatty acids synthesis activity but could enhance the activity at low concentrations.
2. WT, F-TesA, FabG, FabI, FabZ and M-TesA, FabG, FabI, FabZ
FabG, FabI, FabZ play significant roles in the elongation of carbon chain of fatty acyl-ACP. We tried to decrease the distance between the product by linking enzymes to aggregated membrane proteins in order to accelerate the reaction and increase the turnover per unit time. This would finally make it possible to efficiently produce fatty acids with longer carbon chains which are much more preferable biofuel precursors.
We combined these two strategies together to optimize the productivity of the system we established. Notable increase in both diversity and amount of fatty acids were detected, which lends strong support to our hypothesis.
Future Direction
Our study indicates a promising application prospect in biofuel production and further refinement could make it even brighter. FatA is another candidate thioesterasederived from Arabidopsis thaliana. The employment of C18-specific FatA enables the engineered biosynthesis system to produce fatty acids with even longer carbon chain.
|  "
"
