Team:SJTU-BioX-Shanghai/Project/project1.3
From 2012.igem.org
(Difference between revisions)
AleAlejandro (Talk | contribs) (→Background) |
AleAlejandro (Talk | contribs) (→Membrane Rudder) |
||
| Line 33: | Line 33: | ||
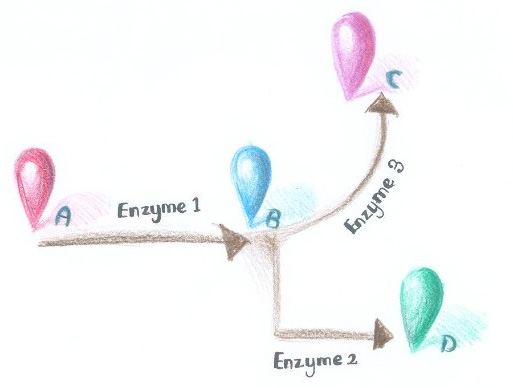
''Membrane Accelerator'' employed constitutively dimerizing proteins to assemble enzymes. Imagine if we replace those constitutively dimerizing proteins with signal-induced dimers, which could only form dimers with signal input, then it is possible to dynamically control the direction of branched reactions. | ''Membrane Accelerator'' employed constitutively dimerizing proteins to assemble enzymes. Imagine if we replace those constitutively dimerizing proteins with signal-induced dimers, which could only form dimers with signal input, then it is possible to dynamically control the direction of branched reactions. | ||
| - | + | There are indeed many signal induced dimers that commonly exist in nature. | |
| - | + | ||
| - | + | ||
| - | + | ||
<br> | <br> | ||
| Line 43: | Line 40: | ||
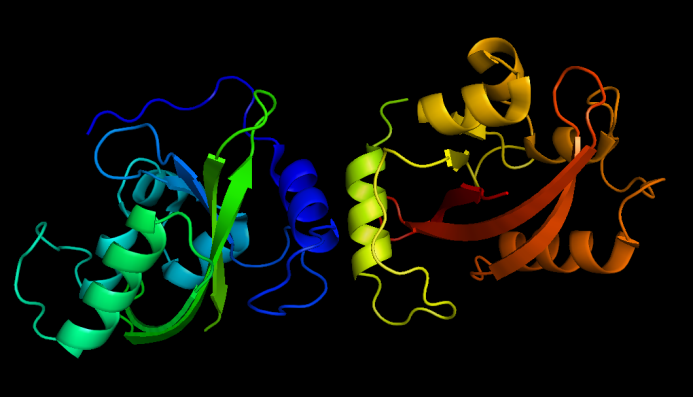
[[Image:VVD dark state.png|thumb|320px|left|''Fig.8'' :Dark state VVD dimers, from PDB ID 2PD7]] | [[Image:VVD dark state.png|thumb|320px|left|''Fig.8'' :Dark state VVD dimers, from PDB ID 2PD7]] | ||
| - | Light is an intriguing signal to regulate ''E.coli'' activity because it is easy to obtain, highly tunable | + | Light is an intriguing signal to regulate ''E.coli'' activity because it is easy to obtain, highly tunable and nontoxic. A light-switchable system could be quite fascinating. |
| - | + | Vivid(VVD) protein, photoreceptor of ''Neurospora crassa'' can form dimer in the presence of blue light and disassociate as light is off. Besises, VVD protein belongs to the Per-Arnt-Sim(PAS) protein superfamily. | |
| - | |||
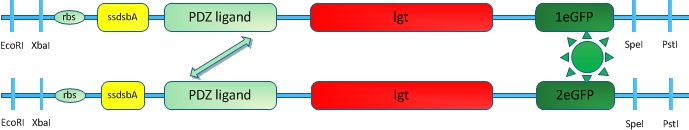
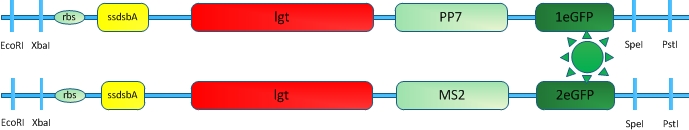
[[Image:12SJTU_VVDTEST.jpg|thumb|320px|right|''Fig.9'' :Fusion protein constructed to test the usability of VVD protein in our membrane system]] | [[Image:12SJTU_VVDTEST.jpg|thumb|320px|right|''Fig.9'' :Fusion protein constructed to test the usability of VVD protein in our membrane system]] | ||
| | ||
| Line 75: | Line 71: | ||
Recruiting those signal induced dimers could easily broaden the application field of our system. What we offer is just a universal tool which can be modified for different use. | Recruiting those signal induced dimers could easily broaden the application field of our system. What we offer is just a universal tool which can be modified for different use. | ||
| + | ==Summary== | ||
<!----------------------------------------------------end---------------------------------> | <!----------------------------------------------------end---------------------------------> | ||
{{Template:12SJTU_footer}} | {{Template:12SJTU_footer}} | ||
Revision as of 20:41, 26 September 2012
 "
"