Team:SEU O China/Project
From 2012.igem.org


Project
Background
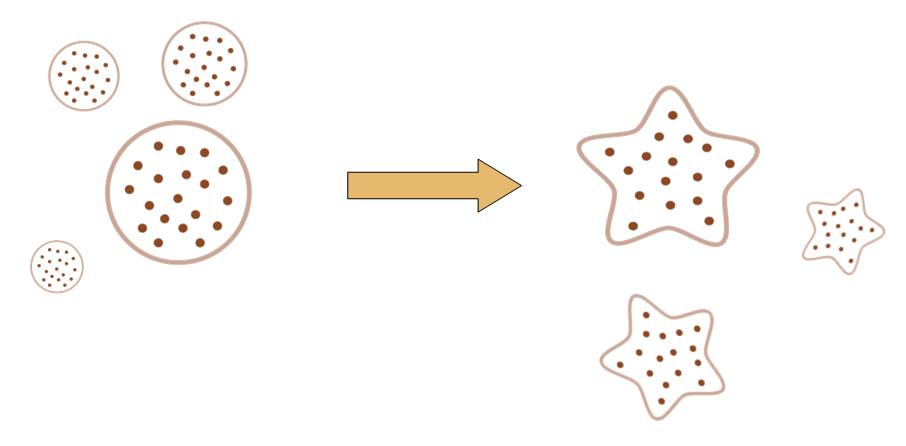
The original idea of this project is really simple. Getting bored of the indifferent, round round colony on the culture dish, we wish to make the bacteria grow into the form of a star, a smiling face, or any other patterns we desire.
Instead of the traditional method of painting a star on the culture dish, and let the colony grow into this pattern, we try to design a biobrick system which let the bacteria take the irregular shape all by itself, despite external conditions.
That means, not only the colony can grow into the shape of a star, but also, even if you pick one cell from the colony, and incubate it on another clean culture medium. That cell will also grow into a colony, which has the shape of a star.
Although the idea is like some kind of bio-game, our project is not that meaningless. Comparing the single cell organism like Escherichia coli, and some simplest multicellular organism like Slime which can act like a whole body in tough environment, we notice that a significant difference between them is that the cells of a multicellular organism can act as a whole, and construct a certain structure.
That is to say, the biobrick system we are constructing is meant to make the single cell bacteria grow into a colony which can act like a multicellular organism. To perform gene manipulation on a multicellular level would be a promising and interesting task.
Story
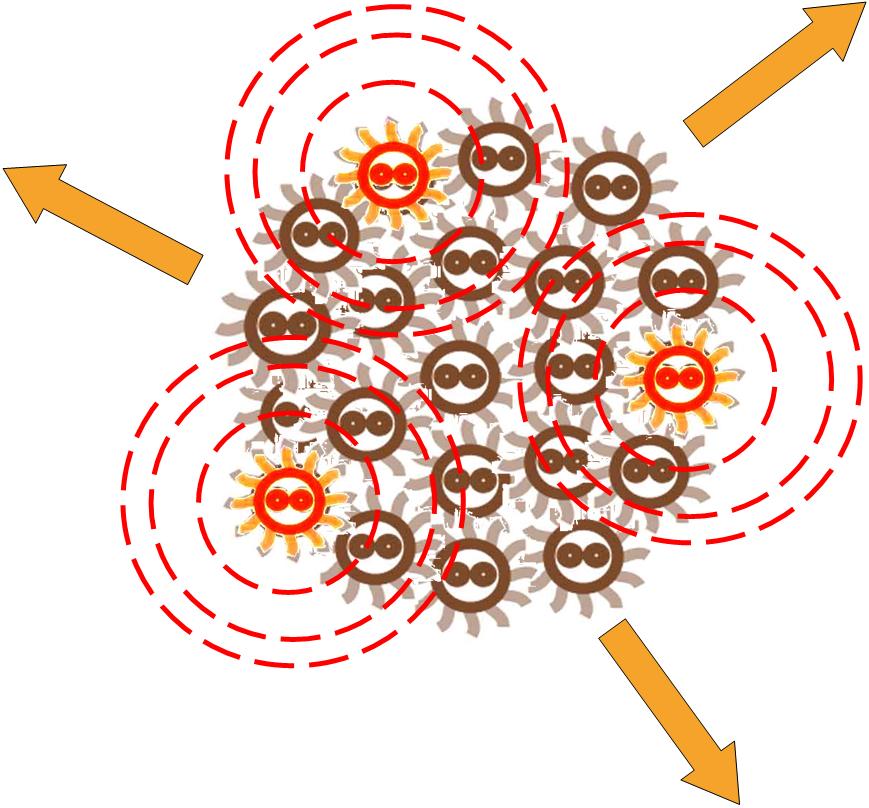
At first, the cells in a colony are all indifferent, and the toggle switches in cell are on the same status.
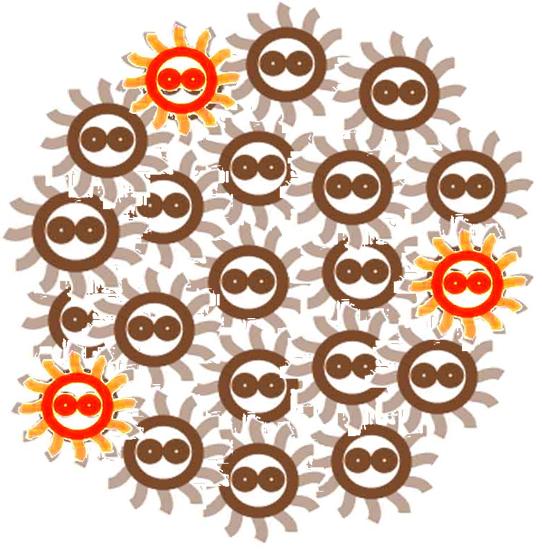
After some events, some of the cells become special. The switch is turned on, and the differentiation happens. The special cells can act like a 'brain', and control the behavior of other cells.
The special cells generate signal molecular which affect the nearby cells in the colony.
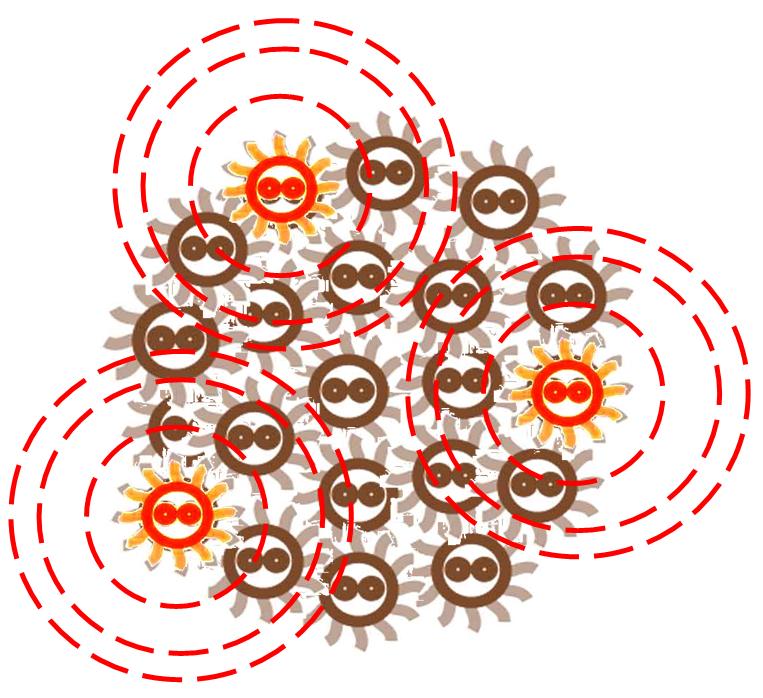
Responding to the density of signal molecular, the division rate of cells in the colony changes differently. Bacteria tend to divide slower in the place where the signal density is higher.
After some time, the Bacteria colony would grow into a certain pattern like this.
By controlling related parameters, it would be possible to create different shapes with bacteria colony.
The very idea of this process (and even the name of our project) comes from the slime's fruit body generation, which includes the formation of special regions inside colony, the intercellular communication, and the control of cell movement[1]. Special thanks to those one-level monsters we have fought in so many games for years....
[1] Oleg A. Igoshin et al. Breaking symmetry in myxobacteria, Curr Biol. 2004 Jun 22;14(12):R459-62.
System
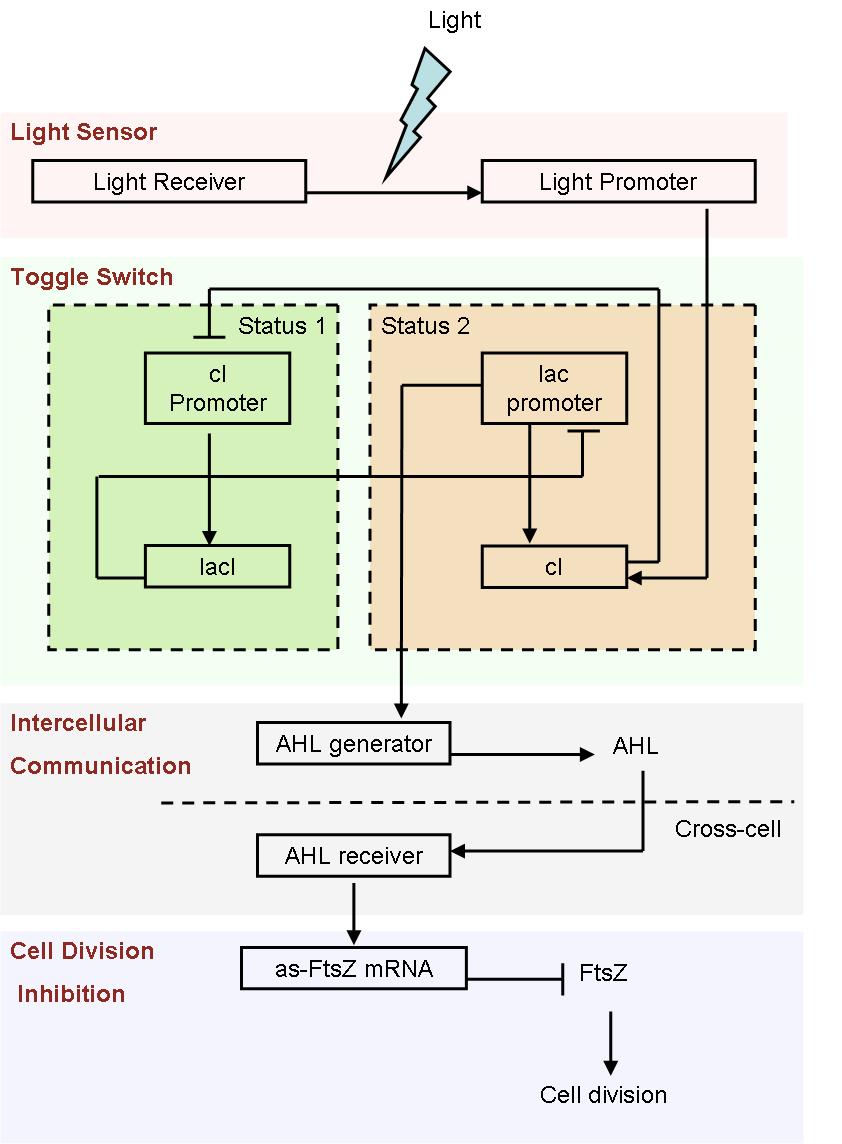
In order to construct a multicellular system, several vital parts must be taken into consideration. We use a toggle switch to save a status, which simulates the cell differentiation, a AHL generate-receive system for intercelluar communication, an antisense mRNA biobrick as cell division inhibitor, in order to control the shape of bacteria colony. We designed a cell movement control device to start the differentiation automatically, as well as a light sensor system to start it artificially for easier control and further application.
Since it is not easy to create a auto-differentiation system from scratch, we decide to start from a light induced system. That means, the first special cells are triggered artificially by light.
- Fig1. Scheme of light induced differentiation system:
At the beginning, we can set all the cells are on status 1 by exposure of UV light. Thus, the cI promoter is switched on and lacI protein is produced. So, at this time, the lac promoter is inhibited and the signal molecular is not generated.
When a cell gets a light signal, the light sensor system will be triggered, and a small amount of cI protein will be produced, which can repress the cI promoter. Then, the toggle switch will be set to status 2, in which the lac promoter is active. Promoted by the lac promoter, AHL molecules are generated from those special cells.
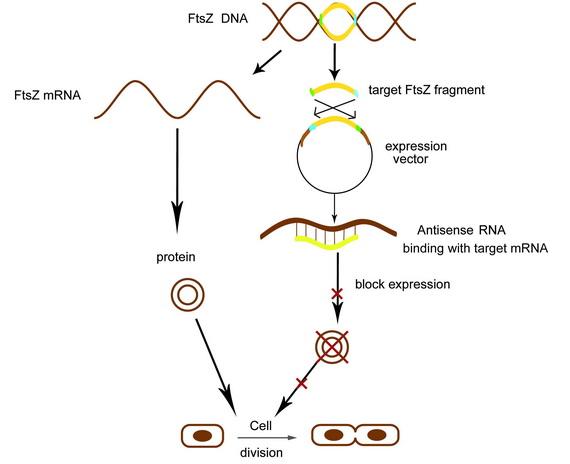
Receiving the AHL molecules, cells start their division inhibition system. An antisense mRNA is transcript, which can bind the mRNA of an essential cell division protein, FtsZ, and thus inhibit its expression and repress the growth of that part of colony. As a result, by controlling the position of light signal, we could create an unsymmetrical colony.
The simulation of this system can be seen here:
and
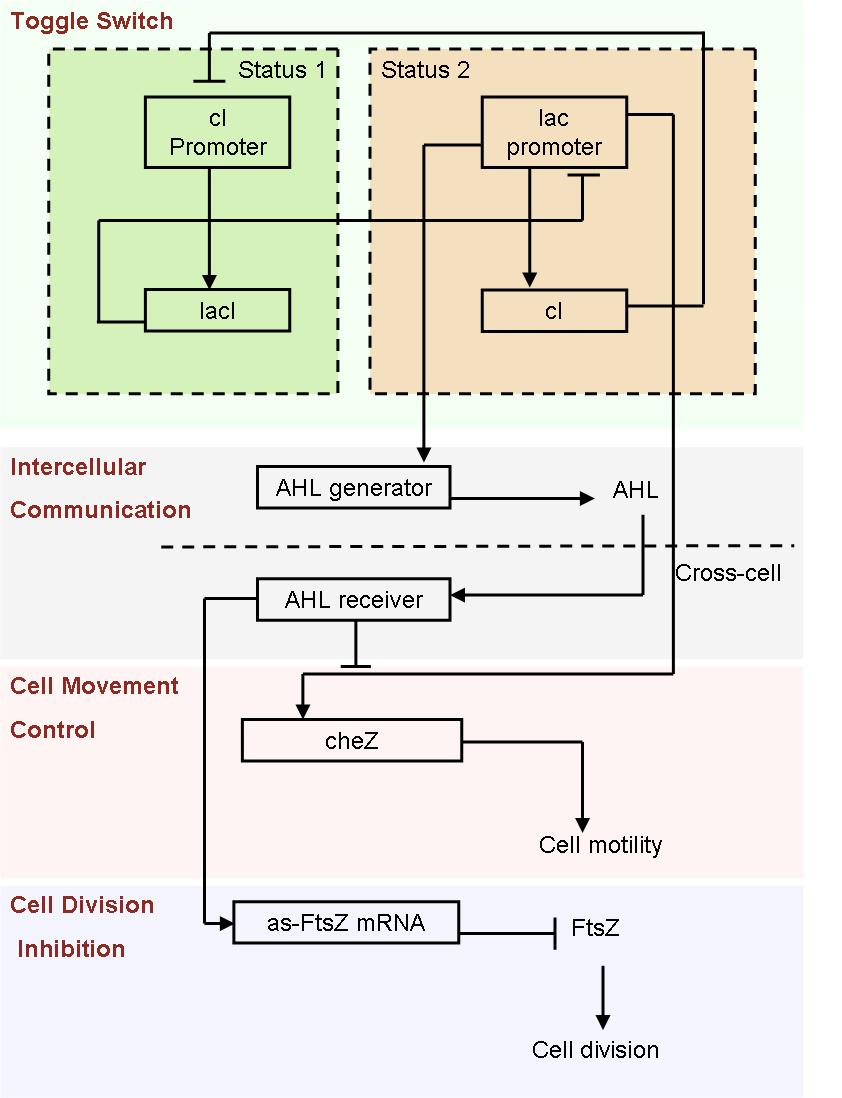
Based on the light induced system, we started to design an auto-differentiation system.
- Fig2. Scheme of auto-differentiation system:
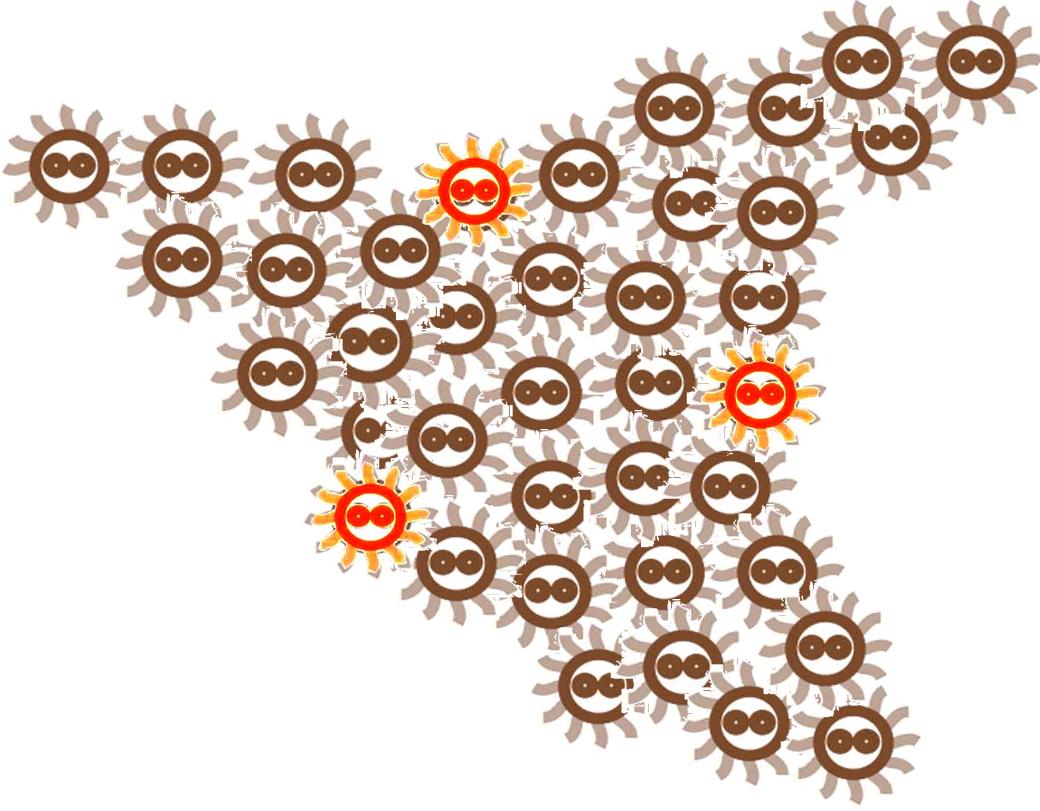
Without external control, the toggle switches in cells start on random status and the rate between status 1 cells (green cells) and status 2 cells (red cells) is certain.
Same as the light induce system, the red cells start to produce AHL molecules. However, the difference is that, in this system, red cell tend to move while the AHL density is high, and stand still while low. Thus, the red cells can move toward the high AHL density region. Since the red cells themselves are AHL sources, this behavior leads red cells gather into several clusters inside the colony.
These red cell clusters, just like the light induced cells in the last system, generate signal molecules and control the division rate of other cells nearby. In the same way, an unsymmetrical pattern may appear on the culture dish.
The simulation of this system can be seen here:
Light Sensor
Light sensor is one of prime parts in our whole project and is constructed by two different scheme,considering the multiple choices of light source and reliablity of those two scheme.
Red light
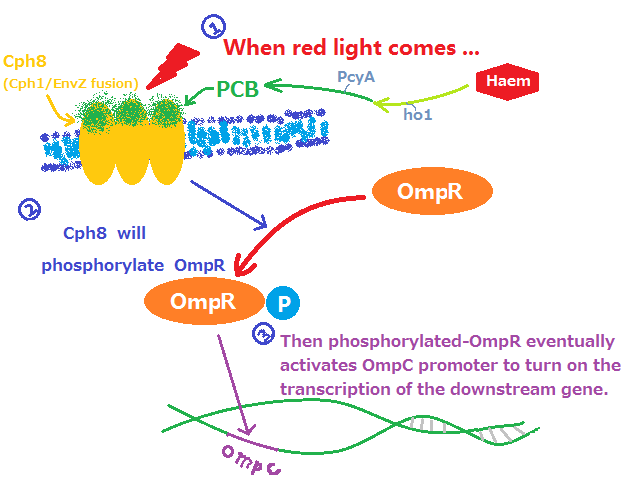
This functional device,allowing the expression of a certain gene to be regulated by red light, consists of two main parts: phytochrome part and regulation part. phytochrome part is responsible for light sensing and regulation part converts the light signal in gene regulational signal.
- Fig1. Red light receptor system
Phytochrome part is a two-component system that consists of a membrane-bound, extracellular sensor (PCB) and an intracellular response-regulator (Cph1). Phycocyanobilin, abbreviated as PCB,is conversed from haem through a series of biological reactions that are catalyzed by heme oxygenase (ho1, BBa_I15008) and ferredoxin oxidoreductase (PcyA,I15009). It acts like an antenna and responds to light as many other members in the phytochrome family. However,phytochromes usually lack DNA-binding domains and cannot regulate gene expression, which requires Cph1 to response and pass on the light signal to regulation part. Cph1, also known as synechocystis phytochrome, is combined with the histidine kinase domain of EnvZ which is one of the key components in EnvZ-OmpR system. The chimaera of Cph1 and EnvZ is named as Cph8(BBa_I15010) that is marked in yellow in Fig.1.
Regulation part is mainly based on EnvZ-OmpR systems. Once activated by the light signal from Cph1, the EnvZ in Cph8 phosphorylates the endogenous OmpR, a transcription factor that results in the expression of the gene after the OmpC promoter. It is noteworthy that endogenous EnvZ should be knocked off from the E.coli strains, which ,if not,may cause the continuous expression of gene after OmpC.
BBa_M30109 is expected to be a reliable kit while on the contrary, we failed to get the correct sequencing report of BBa_M30109. It also reminds us there might be some unsure problems like plasmid size causing the failure of BBa_M30109. As a result,we started constructing two relatively small plasmids: RSB, consisting of BBa_R0010, BBa_S03419 and BBa_B0015, and JIK, consisting of BBa_J13002, BBa_I15008 and BBa_K081024.
Up to now,after combined BBa_S03419 with BBa_B0015 and K081024 with I13507 (a report protein,RFP), we unexpectedly found that the strains of E.coli we use has endogenous EnvZ and until now we realized that EnvZ commonly exists in the genome of most E.coli strains. That's why we emphasize the importance of lack of endogenous EnvZ in the above.
Blue light
Blue light sensor is relatively more simple but more reliable compared with red light sensor. We now start explaining how it works from the key promoter:BBa_K238013.
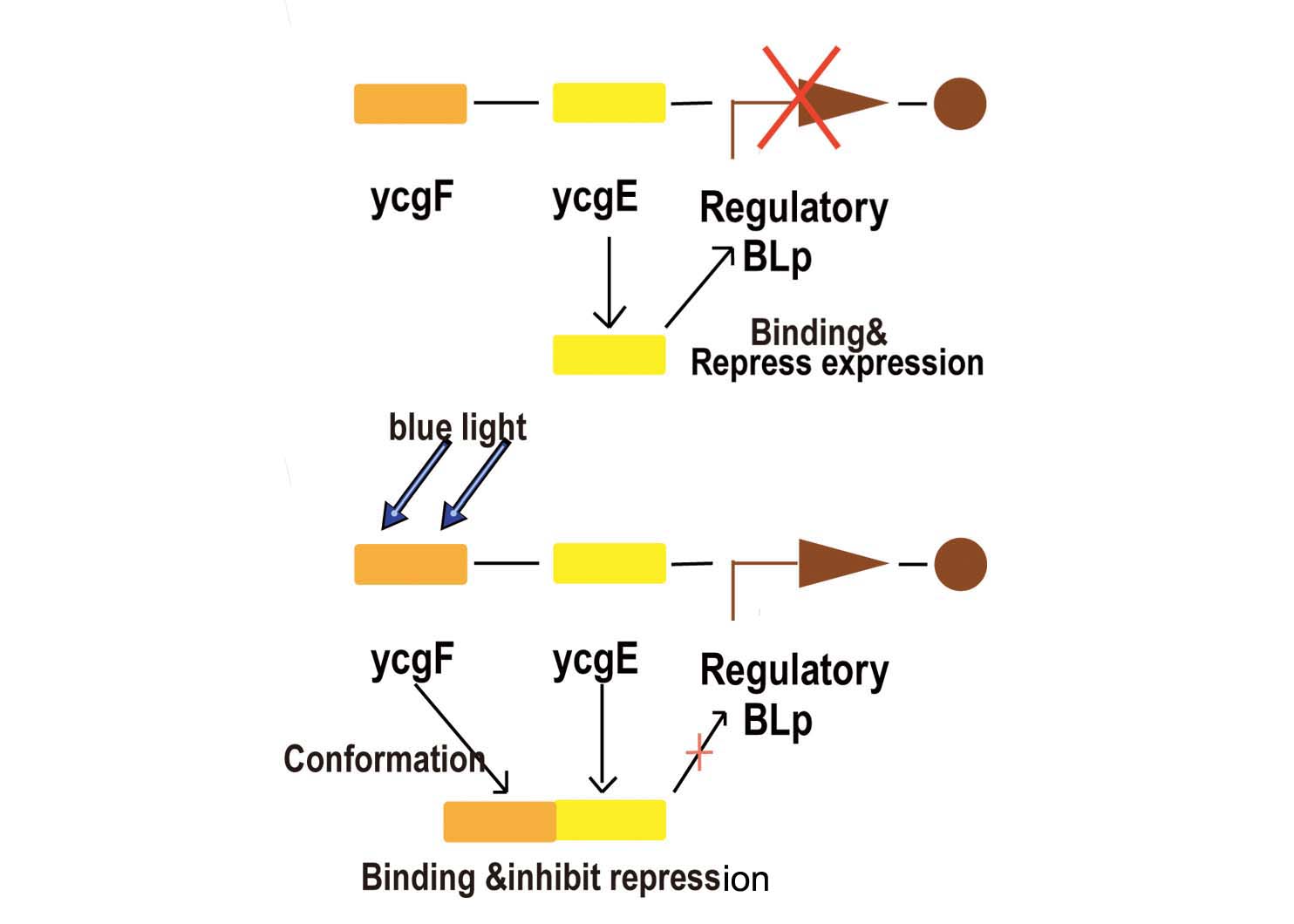
- Fig2. Blue light receptor system
Part BBa_K238013 is a promoter which normally lies before the ycgZ gene, a part of an operon consisting of several genes used by E.coli for regulating e.g. biofilm production. It is regulated by the ycgF/E system. This system consists of a receptor, ycgF, which is responsive to blue light. When blue light strikes, the receptor changes conformation and dimerizes. This allows it to bind to the ycgE repressor through its EAL-domain, releasing the repressor from the promoter region. The inverted repeats shown here are expected to represent the binding site for ycgE. YcgE binding results in transcription repression.
As the experiments done by team iGEM09_KULeuven shown, aside from light, temperature is a very important influence (if not a bigger one). for instance when working in low copy plasmids temperature will influence the promoter/repressor ratio. Cells are grown at 37°C will have too much promoter resulting in constitutive activity. At 16°C, there will be more repressor and receptor but the low temperature will put the receptor in its active state thus inhibiting the repressor from doing its job. When grown at 25°C the ratio is better and the receptor/repressor are still in there ground state leading to a good repression of the promoter.
Most fortunate for normal transgenic work of this light sensing part is that the ycgF/E system is a necessary for competent cells like BL21 or DH5α, which means extra implant of the ycgF/E system into competent cells is unnecessary.
We, by now in our project, have tried to use this blue light sensing system as a switch in the first step. Once triggered, it would lead to the execution of continual procedures.
Moreover, in an effort to improve biosafety standards in modern synthetic biology research, we have been working to construct a new biobrick that functions as a light-sensitive division repression tool. So long as the culture dish is kept under normal light condition, the implanted genes would not express at high colony densities.
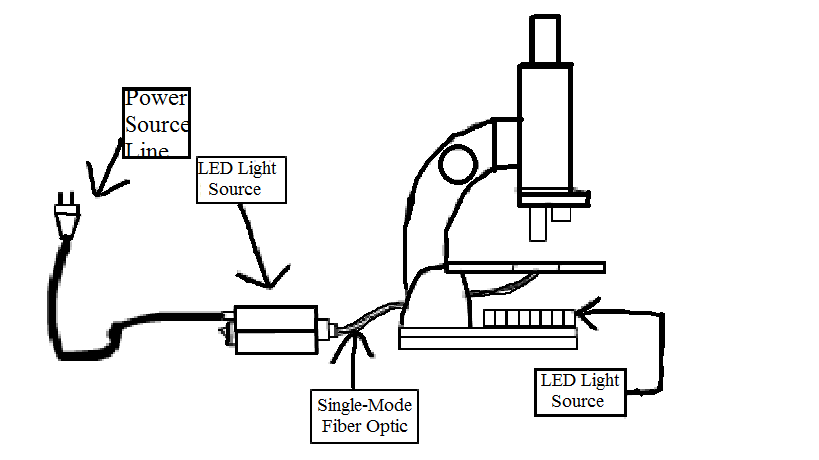
Light-inducing system In order to induce several cells in a colony with light, we designed a single-mode fiber based device for micro-scale operation.
- Fig3: light-inducing system
The figure displays the basic principle of light-inducing system based on single-mode fiber optic.
Some special modulations must be made on the normal microscope.The original reflection mirror is changed into a LED light source array that would be used in dark environment to lit the target. As this device aims to provide a special light inducing condition, two LED light sources should of course provide different lengths of light, for example, blue light and red light. In order to accurately targeting colony on a micronmeter level. A special fiber optic line would be used to minimize the light inducing diameter to at most 65 micronmeter. The light source has a special interface to the fiber optic so it has to be specially made.
References
Anselm Levskaya et al. Synthetic biology: Engineering Escherichia coli to see light. Nature,November 24,2005.438:441-442
Jeffrey J.Tabor et al. A Synthetic Genetic EdgeDetection Program. Cell,June 26,137: 1272-1281
Toggle Switch
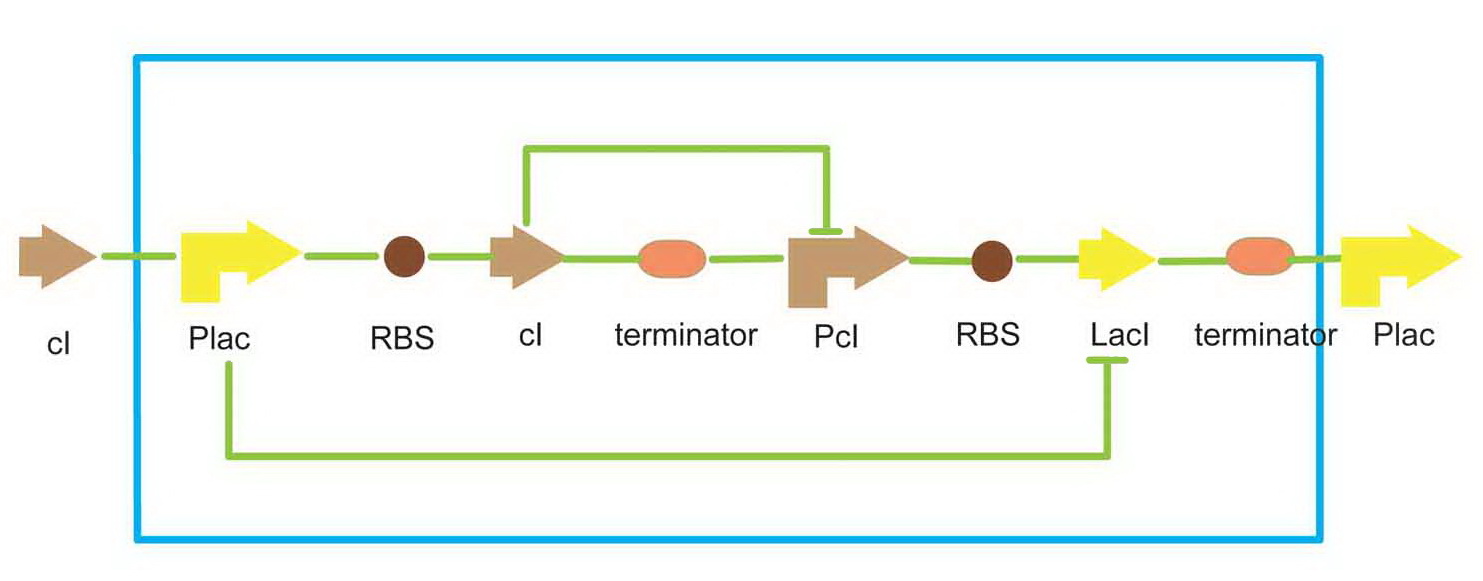
We use a traditional lacI-cI bi-repressor for toggle switch.
- Scheme of toggle switch
There are two promoters in this toggle switch, the plac, which is inhibited by lacI protein, and pcI, inhibited by cI protein. That means, the two passageways, plac-cI and pcI-lacI, can repress each other. Just like the Bistable Multivibrator(or flip-flop) in digit circuit, only one passageway can be activated at one time in a cell. This equipment enable a cell to 'remember' a status, and behavior differently in each state. As a result, we can have cells in a colony that have the same genetype but different phenotype. We use this toggle switch to simulate the function of cell differentiation in the project.
The toggle switch can be set to the pcI-lacI state by exposure of UV light. When the system upstream, which is the light sensor system in our project, is activated, an amount of cI will be produced, which can repress the cI promoter. Then, the toggle switch will be set to the plac-cI state. Since plac is turned on, the downstream gene can be expressed.
References:
[1] Hideki Kobayashi et al. Programmable cells: Interfacing natural and engineered gene networks. PNAS, April 26, 2004
Intercelluar Communication
An AHL generate-receive system is used for our project.
Introduction
These are small signalling molecules which are employed in "quorum sensing" systems. They are also known as autoinducers (AIs) and are present in many Gram-negative bacteria.
"Although the target genes regulated by AIs are extremely varied, the basic mechanism of AIs biosynthesis and gene regulation seems to be conserved in different bacteria. The general feature of gene regulation by AIs is cell-density dependence, also known as "quorum sensing".
At low cell densities, the AIs are at low concentrations, and, at high cell densities, the AIs can accumulate to a concentration sufficient for activation of related regulatory genes. Because the concentration of AHL's is a key factor in determining virulence gene expression in several pathogenic bacteria, it is possible to develop a strategy for disease control by controlling production of AIs or eliminating AIs produced by pathogenic bacteria."
AHL binds to the protein product of the LuxR gene and activates it. The C-terminal domain of activated LuxR relieves the repression exerted by H-NS nucleoid proteins that bind to the promoters of LuxR, LuxI and the LuxCDABEG operon, as well as to A-T-rich stretches within that operon and other genomic regions. The product of LuxI catalyses the synthesis of AHL. Thus, AHL acts as an autoinducer. Transcription of the LuxCDABEG operon results in luminescence due to the expression of LuxA and LuxB, which form a protein known as a luciferaseand the expression of LuxC, D, E, and G, which are involved in the synthesis of the luciferase's substrate, tetradecanal. This is an important feature of quorum sensing, as it makes little sense for one cell to waste the energy producing light, as the resulting light will be so faint that it will be more or less undetectable. Instead, once the bacterial population has reached a specific size, only then does light production commence.
The auto-inducing and quorum-sensing related feature plays a rather significant role in the design of our scheme. In order to manipulate the division rate due to different density of cells, we tried to construct a cell-cell signaling pathway of AHL for communication. The information carried by AHL would accumulate and trigger the toggle switch when reaching a threshold value.
Sender devices
Currently, we chose a sender device BBa_F1610 as a device that accepts an input of PoPS and outputs a rate of signaling molecule synthesis.
Receiver devices
We chose a receiver device BBa_F2620 and its related composite part BBa_T9002 as a set of genetic elements that responds to an extracellular input signal by changing the PoPS output from the device.
The whole AHL pathway would serve as pathway to send signal to the continual parts. However, due to a number of reasons, this system has been realized during our summer session.
Reference
[1] http://partsregistry.org/Featured_Parts:Cell-Cell-Signaling;
[2] http://partsregistry.org/wiki/index.php/Part:BBa_F2620;
Cell Division Inhibitor
Introduction
Aiming at creating an asymmetric colony, we use the antisense FtsZ sequence to silent the FtsZ gene and control the self-renewal and differentiation of E.coli. We create a kit which can receive AHL signaling and promote the antisense sequence to repress the division of prokaryotes.
FtsZ
To fabricate this part, we decided to utilize the FtsZ gene to manipulate the division of E.coli. Cell wall division in E. coli involves a complex series of events with the involvement of at least eight different proteins. One of the first steps in bacterial cell wall division involves the formation of the Z-ring, a circular polymeric structure formed by the cytosolic protein FtsZ.
FtsZ is a prokaryotic homologue of andα- andβ-tubulin in E.coli. And like tubulin, it conducts GTPase and polymerization activities in vitro. The exact structure of the Z ring in vivo is not known, but there has been evidence proving that it is highly dynamic, being continuously remodeled, whose frequency is almost proportional to the amount of its GTPase activities . And FtsZ can assemble into the Z-ring without the assistance of any protein so far identified.
It has been demonstrated that FtsZ has a precise localization within the bacterium. Immuno-gold electron microscopy showed a diffuse localization in most cells but the Z ring of concentrated FtsZ near the membrane in cells that were about to divide. As division proceeded, the FtsZ ring constricted and remained at the tip of the invaginating membrane.
In conclude, FtsZ is one of the best conserved division proteins in its family and its role in cell division traces back very early in evolution.
- Fig1: Antisense-Ftsz system
Antisense RNA
In order to interfere with the cell division, we synthesize the right antisense fragments with gene silencing effects targeting ftsZ in E.coli.
The antisense inhibition uses single-stranded ODNs (oligodeoxynucleotide), which bind to a specific mRNA via base-pairing. The DNA–RNA duplex that is formed inhibits translation into the corresponding protein by altering mRNA splicing, translation or degradation. A vector with paired termini was constructed to constantly express different asRNAs of ftsZ in E.coli, and the gene-silencing effects were observed by morphological observation on-plate,microscope and liquid culture. This part has been found inhibiting cell division considerbly.After that, we ligated it after the AHL receiver F2620 and let the AHL signaling promote it. Meanwhile, to witness the function of the paired termini, we also constructed series of kits only with the target fragments.
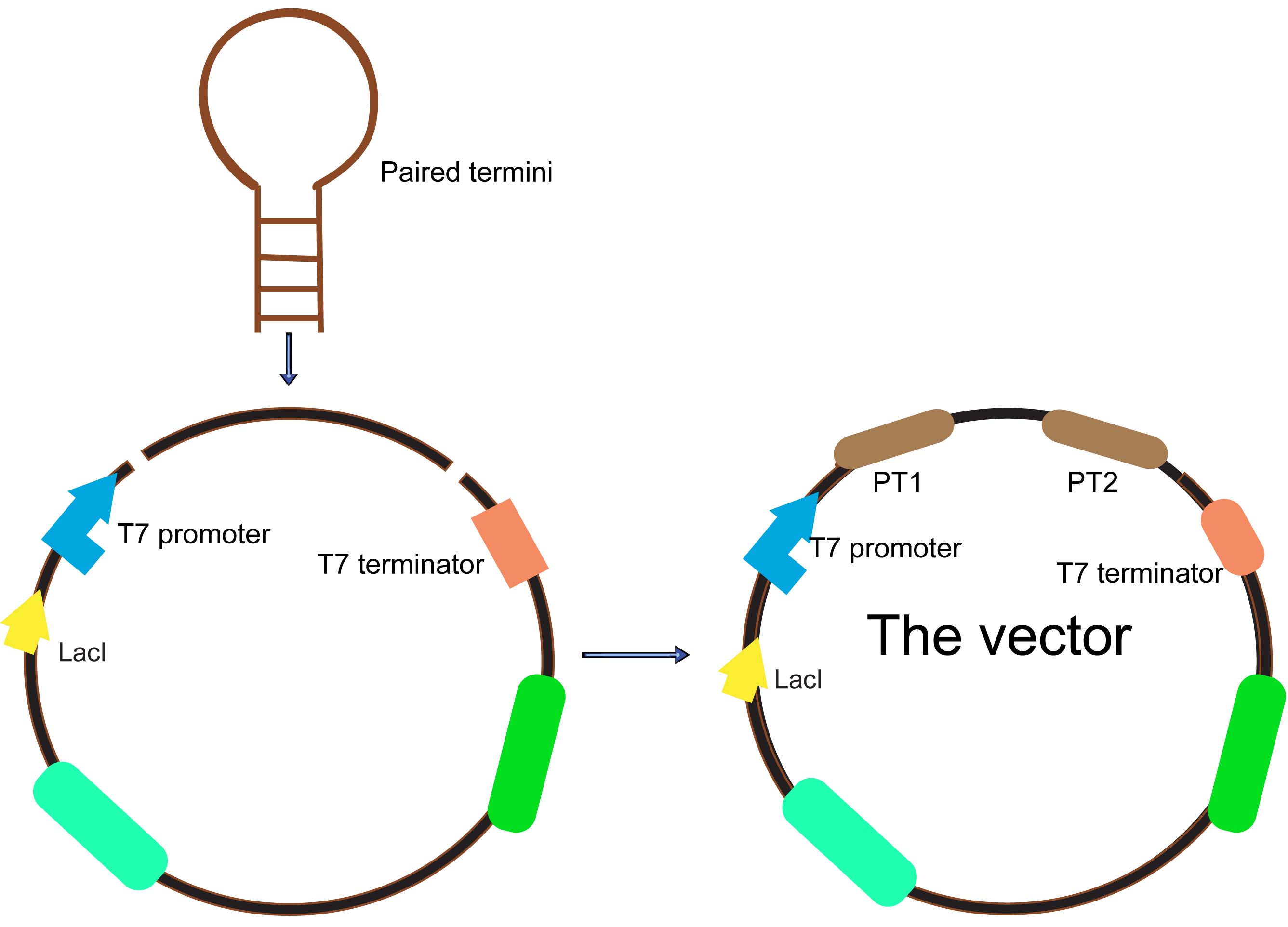
The paired termini
As we know, all antisense RNAs had a short half-life of about 1 minute. Because antisense RNA efficiency is determined by the binding rate to the target, which in turn is determined by both antisense RNA concentration and binding rate constant, a higher intracellular concentration of the inhibitor will result in higher efficacy.
An alternative strategy to stabilize asRNAs is to pair the termini using flanking inverted repeats to create a hairpin structure with the antisense sequence within a large loop. A paired termini (PT) design, where flanking inverted repeats create paired asRNA termini, was proved can produce effective gene silencing. PT asRNAs are abundant and stable and function through an RNase III independent mechanism that requires a large stoichiometric excess of asRNA. PT consists of non-endogenous GC-rich sequences and has a stem-loop structure, which can improve the stability of as RNA raises its abundance.
- Fig2: Paired Termini
Reference
[1] Effect of different antisense RNA sequence on ftsZ gene silencing in Escherichia coli, CHEN Yin,QIAN Xiu-ping,HE Jian-yong,GE Mei,YIN Yu, Journal of Shenyang Pharmaceutical University , Vol. 28 No. 7 Jul. 2011 p. 564
[2] Antisense technology in molecular and cellular bioengineering, Current Opinion in Biotechnology, Volume 14, Issue 5, October 2003, Pages 505-511 , Li Kim Lee, Charles M Roth
[3] Paired termini stabilize antisense RNAs and enhance conditional gene silencing in Escherichia coli, Nobutaka Nakashima, Tomohiro Tamura and Liam Good, Nucleic Acids Research, 2006, Vol. 34, No. 20
[4] Molecular dynamics simulation of GTPase activity in polymers of the cell division protein FtsZ, Fernando Martín-García, Estefanía Salvarelli , Jesús Ignacio Mendieta-Moreno , Miguel Vicente, Jesús Mingorance , Jesús Mendieta, Paulino Gómez-Puertas , F. Martín-García et al. / FEBS Letters,586(2012).
[5] Discovery of novel inhibitors of the ZipA/FtsZ complex by NMR fragment screening coupled with structure-based design, Bioorganic & Medicinal Chemistry 14 (2006) 7953–7961, Desiree H. H. Tsao, Alan G. Sutherland, Lee. D. Jennings, Yuanhong Li, Thomas S. Rush, Juan C. Alvarez, Weidong Ding, Elizabeth G. Dushin, Russell G. Dushin, Steve A. Haney, Cynthia H. Kenny, A. Karl Malakian,Ramaswamy Nilakantana and Lidia Mosyaka.
Application
Preface
If you ever thought our project is nothing more than creating a pattern of triangle or hexagon, than you are wrong; If you ever thought our project is merely a display for this competition, than you are wrong; Or if you ever thought our project is anything but practical, than you are completely wrong. What here I want to emphasize is that the idea of our project initialized from the observance on the development of modern biology and that what we are trying to construct is a part that be manipulated in a number of ways.
Overall View
Evidently, the essence of our project is to break the symmetry of colony and try to acquire a certain pattern.
From the holistic point of view, our project aims to gain a more specific and accurate microarray of cells. For in modern biology, more and more technologies have rather strict requirements for calculated sequences, not only for molecules but also for cells.
First of all, modern biologists have developed cell-printing in order to construct bio-circuits whose function limits to a single terrain of elaborate-designed cells. In addition, the cell culture also requires special treatment. For our design, however, the construction of specific array lies in the induced division alternative behavior of cells, which simplifies the culture condition. Although according to our initial design, complex microstructure would be impossible, the potential is promising as a more compact inducing method.
Moreover, bio-sensor may embrace a new era. Past biosensors, no matter on high-flux molecular level or on cell level, were in fact too dependent on relative precision instruments such as X-ray detraction instruments and spectrophotometers that can only be purchased and applied for by rather high level laboratories. For a more practical prospect, our design is exactly expecting a more direct display of parameters. A specific cell array would lead to a known density of cells and that a clearer transformation of parameter to fluorescent protein would be as simple as a PH testing paper, though not that accurate, but would be more practical.
Last but not least, breaking the symmetry would be meaningful to evolution research. As our original idea stems from the primitive quorum interaction of slime model, we regard the pattern of asymmetry as a very first step to the multicellular organism. The separation of division would accumulatively grow into the separation of functional types. What we are trying to construct is also a primitive differentiation.
Bio-Computer
We are going to take bio-computer as an example to show the application of our project.
Synthetic biology has advanced the design of standardized control devices that program cellular functions and metabolic activities in living organisms1. Rational interconnection of these synthetic switches resulted in increasingly complex designer networks that execute input-triggered genetic instructions.
Bio-computing seems promising in that a cell computing system, compared to silicon chips, would require lower energy-cost. In addition, as solid-state circuits have faced more and more challenges in thermal design, the liquid-state bio-computing technique might be a potential solution.
However, by now, no matter on single-cell or multi-colony level, real diverse and complex calculations have not been plausible due to the limits on the complexity of signal molecules or the difficulty of arranging cells in an appropriate sequence.
With our system, we can create a bio-computer on the single colony level, which may be able to realize the function multiple logic gates or even a simple calculator.
Detailed description and simulation can be found in our model page: https://2012.igem.org/Team:SEU_O_China/Model#cocontent
References
[1] Alvin Tamsir et al. Robust multicellular computing using genetically encoded NOR gates and chemical ‘wires’, Nature 469, 212–215
[2] Simon Ausländer et al. Programmable single-cell mammalian biocomputers, Nature (2012) doi:10.1038

 "
"