Team:Penn/HumanPracticesOverview
From 2012.igem.org
| (16 intermediate revisions not shown) | |||
| Line 16: | Line 16: | ||
<b><div class="name" align="center">Human Practices: Transitioning From the Bench to the Bedside</div></b> | <b><div class="name" align="center">Human Practices: Transitioning From the Bench to the Bedside</div></b> | ||
<br> | <br> | ||
| - | Many previous iGEM teams have tried to implement a bacterial therapeutic as part of their project. Outside of iGEM, there has been a steady interest in engineering bacteria to become therapeutic vectors as well. However, the question that guided our human practices project was essentially: <b>Why aren't bacterial therapeutics transitioning into clinical practice or even clinical trials? </b> | + | <p style="text-align:justify;">Many previous iGEM teams have tried to implement a bacterial therapeutic as part of their project. Outside of iGEM, there has been a steady interest in engineering bacteria to become therapeutic vectors as well. However, the question that guided our human practices project was essentially: <b><br><p style="text-align:center">Why aren't bacterial therapeutics transitioning into clinical practice or even clinical trials? </p></p></b> |
| + | <p style="text-align:justify;"> | ||
| + | While there are certainly many barriers to bacterial therapeutics such as time and money, we hypothesize that iGEM teams, as a result of their unique positions as research and educational institutions, are positioned to address two major barriers to the adoption of bacterial therapeutics: biological barriers and perception barriers. | ||
| + | </p> | ||
<br> | <br> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
</div> | </div> | ||
<div class="bigbox"> | <div class="bigbox"> | ||
| - | <b><div class="name" align="center"> | + | |
| + | <b><div class="name" align="center">Biological Barriers to Bacterial Therapeutics</div></b> | ||
<br> | <br> | ||
| - | <p style="color:black;text-indent:30px;"> | + | <p style="color:black;text-indent:30px;"> |
| - | + | From a technological standpoint, there is a great deal of work that remains to be done before a bacterial therapeutic can enter the drug development pipeline. While many iGEM teams, including us, have helped set the groundwork for bacterial therapeutics, there are still some biological barriers to a bacterial therapeutic. We identified the immunogenicity of laboratory strains of <i>E. coli.</i> as a major biological barrier. We then investigated methods to decrease the immunogenicity of <i>E. coli.</i>, eventually choosing to port modules of our target drug delivery system into a non-immunogenic strain of <i>E. coli.</i>, Nissle 1917. | |
| - | < | + | </p> |
| - | + | <div align="center"> | |
| - | <p style="color:black;text-indent:30px;"> | + | <img src="https://static.igem.org/mediawiki/2012/thumb/c/c6/VerifiGEM-Logo.jpg/800px-VerifiGEM-Logo.jpg" width="900" height="200" /></div> |
| + | <p style="color:black;text-indent:30px;"> | ||
| + | Furthermore, in an effort to speed progress and information sharing between iGEM teams, government research organizations, and private research groups, we have proposed a system known as VerifiGEM that would allow for the quality control of BioBricks to be distributed across the entire iGEM community. Should our system be implemented, the overall quality and reliability of BioBricks will be improved greatly, at very little cost to individual iGEM teams. | ||
| + | </p> | ||
</div> | </div> | ||
| - | |||
| - | |||
<div class="bigbox"> | <div class="bigbox"> | ||
| + | <b><div class="name" align="center">Perception Barriers to Bacterial Therapeutics</div></b> | ||
| + | <br> | ||
| + | <p style="color:black;text-indent:30px;"> | ||
| + | However, the removal of biological barriers to bacterial therapeutics alone is not sufficient to enable bacterial therapeutics to move into the drug development pipeline. Through the course of recent history, many high profile technologies, such as gene therapy or nanotechnology have been met with public skepticism and even fear, as the technologies failed to deliver on earlier promises. This prompted a constriction of available funding and subsequently impeded progress in those fields, an outcome that nanotechnology in particular is only just beginning to recover from. | ||
| + | </p> | ||
| + | <p style="color:black;text-indent:30px;"> | ||
| + | We propose a model, adapted from Gartner Inc, which proposes that the disconnect between the expectations of the public and the realities of scientific research produces an initial "peak of inflated expectations" (and funding), that rapidly disappears as promised advances are delayed or do not work as planned (Figure 1). We believe that this "trough of disillusionment" is the cause of restrictions in funding and a general stall of scientific progress in a given field. We propose that the peak can be smoothed out, reducing the size of peak, but more importantly, eliminating the trough of disillusionment (Figure 2). | ||
| + | </p> | ||
| - | < | + | <div class="figs2"> |
| - | <br> | + | <div class="fignew"><div align="center"><img src="https://static.igem.org/mediawiki/2012/7/7c/Hype-Cycle-Original.jpg" height="200" width="320"><br> |
| + | <b>Figure 1</b></div><div style="text-align:center;">Figure 1: The hype cycle without the modulating effects of public outreach efforts.</div></div><br> | ||
| - | < | + | <div class="fignew"><div align="center"><img src="https://static.igem.org/mediawiki/2012/a/ab/Hype-Cycle-Original---Copy.jpg" height="200" width="320"><br> |
| + | <b>Figure 2</b></div><div style="text-align:center;">Figure 2: The modulation of the extremes of the hype cycle through public outreach efforts such as iGEM. </div></div></div><br> | ||
| - | <p style="color:black;text-indent:30px;"> | + | <p style="color:black;text-indent:30px;"> |
| + | What then, must we as synthetic biologists do to prevent this fate from befalling our own field of study? Certainly, scientists must perform a balancing act between reporting the advances they have made and making realistic conclusions. iGEM teams in particular have a unique opportunity to impact this cycle. Each time a team teaches a class to younger students, presents their research, or even sets up a fun experiment at a local science event, they are given an opportunity to communicate the potential and the limitations of synthetic biology. This is an opportunity many researchers do not have, and should be treated as more than simply a time to take pictures and have fun (although those are certainly important parts of these events nevertheless). | ||
| + | </p> | ||
</div> | </div> | ||
</body> | </body> | ||
</html> | </html> | ||
Latest revision as of 02:21, 27 October 2012
Many previous iGEM teams have tried to implement a bacterial therapeutic as part of their project. Outside of iGEM, there has been a steady interest in engineering bacteria to become therapeutic vectors as well. However, the question that guided our human practices project was essentially:
Why aren't bacterial therapeutics transitioning into clinical practice or even clinical trials?
While there are certainly many barriers to bacterial therapeutics such as time and money, we hypothesize that iGEM teams, as a result of their unique positions as research and educational institutions, are positioned to address two major barriers to the adoption of bacterial therapeutics: biological barriers and perception barriers.
From a technological standpoint, there is a great deal of work that remains to be done before a bacterial therapeutic can enter the drug development pipeline. While many iGEM teams, including us, have helped set the groundwork for bacterial therapeutics, there are still some biological barriers to a bacterial therapeutic. We identified the immunogenicity of laboratory strains of E. coli. as a major biological barrier. We then investigated methods to decrease the immunogenicity of E. coli., eventually choosing to port modules of our target drug delivery system into a non-immunogenic strain of E. coli., Nissle 1917.

Furthermore, in an effort to speed progress and information sharing between iGEM teams, government research organizations, and private research groups, we have proposed a system known as VerifiGEM that would allow for the quality control of BioBricks to be distributed across the entire iGEM community. Should our system be implemented, the overall quality and reliability of BioBricks will be improved greatly, at very little cost to individual iGEM teams.
However, the removal of biological barriers to bacterial therapeutics alone is not sufficient to enable bacterial therapeutics to move into the drug development pipeline. Through the course of recent history, many high profile technologies, such as gene therapy or nanotechnology have been met with public skepticism and even fear, as the technologies failed to deliver on earlier promises. This prompted a constriction of available funding and subsequently impeded progress in those fields, an outcome that nanotechnology in particular is only just beginning to recover from.
We propose a model, adapted from Gartner Inc, which proposes that the disconnect between the expectations of the public and the realities of scientific research produces an initial "peak of inflated expectations" (and funding), that rapidly disappears as promised advances are delayed or do not work as planned (Figure 1). We believe that this "trough of disillusionment" is the cause of restrictions in funding and a general stall of scientific progress in a given field. We propose that the peak can be smoothed out, reducing the size of peak, but more importantly, eliminating the trough of disillusionment (Figure 2).
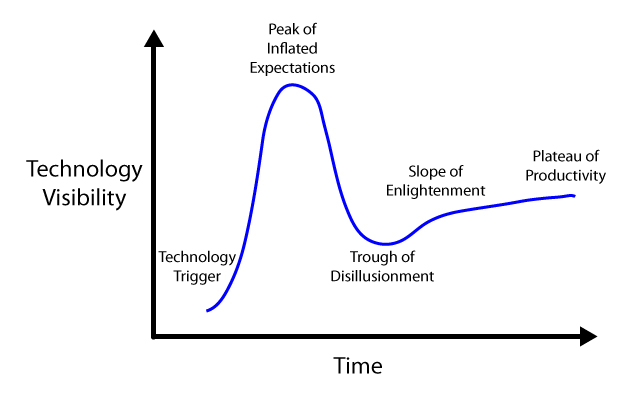
Figure 1

Figure 2
What then, must we as synthetic biologists do to prevent this fate from befalling our own field of study? Certainly, scientists must perform a balancing act between reporting the advances they have made and making realistic conclusions. iGEM teams in particular have a unique opportunity to impact this cycle. Each time a team teaches a class to younger students, presents their research, or even sets up a fun experiment at a local science event, they are given an opportunity to communicate the potential and the limitations of synthetic biology. This is an opportunity many researchers do not have, and should be treated as more than simply a time to take pictures and have fun (although those are certainly important parts of these events nevertheless).
 "
"

