Team:Paris Bettencourt/Suicide
From 2012.igem.org
Contents |
Overview
Our goal is to engineer a synthetic toxin-anti-toxin system from the wild type Colicin E2 (Col E2). This synthetic toxin-anti-toxin system is receptor specific, allows for delayed population-level suicide, complete genome degradation, and will function on a tunable delay.
Background
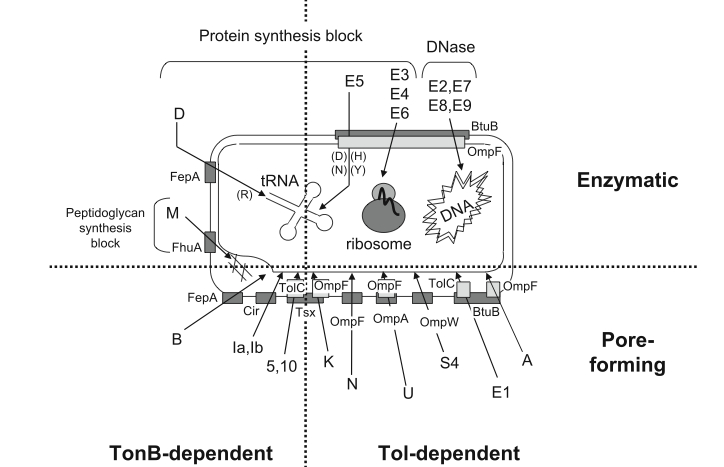
What are Colicins? Colicins are a type of toxic protein produced by strains of E.coli, and are a type of bacteriocin [1]. In natural systems the colicin operons are regulated under the SOS promoter.
Image taken from Cascales E, et al. (2007) Colicin biology. Microbiol Mol Biol Rev 71:158–229
Bigger Picture
Our anti-toxin plasmid can be combined with the restriction enzyme system to generate a toxin-anti-toxin system that works on a tunable delay depending on the induction of the restriction enzyme.
Objectives
- Design an anti-toxin part from the Col E2 immunity gene that confers immunity against the Col E2 activity protein
- Measure viability of immune and vulnerable NEB turbo and MG1655 Z1 cells in the presence of WT Col E2 cells
- Design a Col E2 activity protein part under the control of a constitutive promoter and establish in immune NEB turbo and MG1655 Z1 cells to generate synthetic Col E2 cells
- Measure viability of immune and vulnerable NEB turbo and MG1655 Z1 cells in the presence of synthetic Col E2 cells
- Combine our system with the Restriction Enzyme System, measure viability
Design
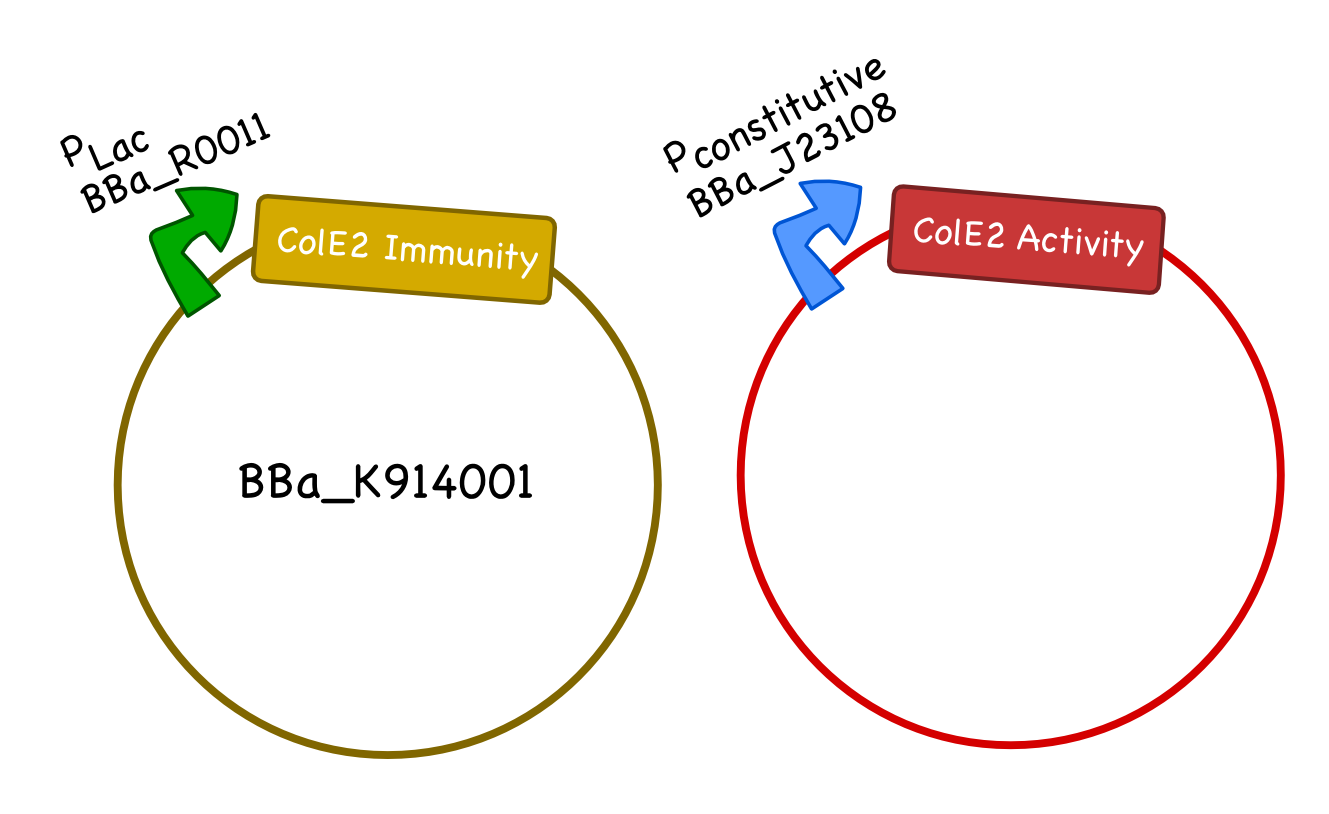
For our system we require two plasmids, one carrying the Col E2 activity protein and one carrying the Col E2 immunity protein. As the immunity protein is normally present in excess in natural colicinogenic cells, we chose a 10-12 copy vector pSB3C5 to carry the immunity protein, and a lower ~5 copy vector pSB4K5 to carry the toxin protein. We used pLac to drive expression of the immunity protein, however, different inducible promoters should be used depending on the overall design and application of the safety circuit. We chose the constitutive promoter BBa_J23108 from the Anderson Promoter Collection, which has a relatively moderate expression level, so that the activity protein would not overwhelm the cells until after the desired delay.
Experiments and results
Characterization of the Colicin E2 Toxicity
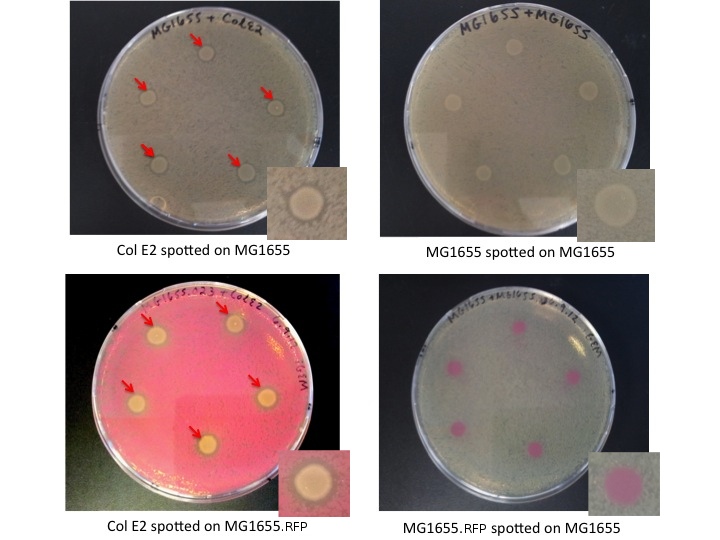
We performed a basic assay to characterize the toxicity of the Col E2 cells. Here we expect zones of inhibition (ZOI, clear rings around the colicin cells) when Col E2 is spotted on vulnerable cells.
Experimental setup
Cell Types
- MG1655: Wild Type E.coli Cells
- MG1655.023: MG1655 cells transformed with constitutive RFP (from Anderson promoter library, BBa_J61002)
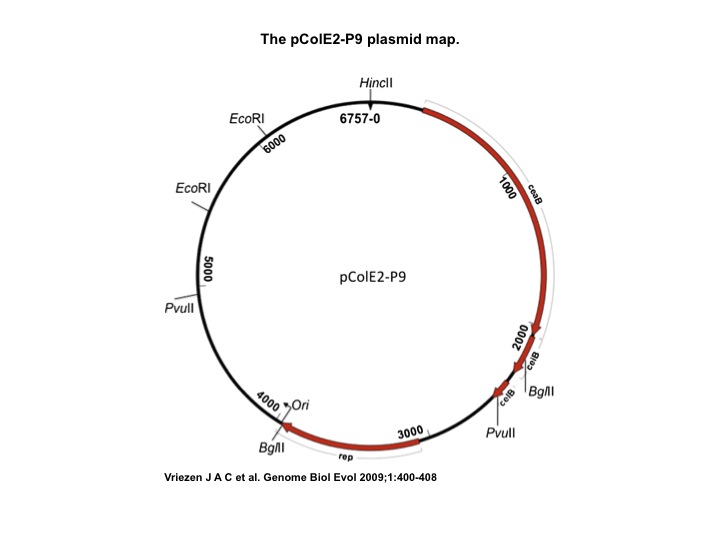
- Col E2: Wild Type Colicin E2 cells, containing pColE2-P9 plasmid [2]
Protocol
- Plate 10 uL of 0.1 OD lawn cells from liquid culture on plain LB plates
- Spot 5 uL of saturated Colicin E2 cells from liquid culture on plates
- Incubate plates overnight
Results
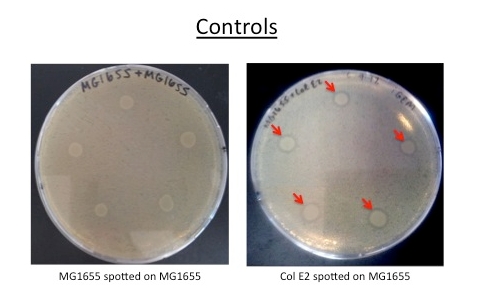
Col E2 cells produce ZOI in vulnerable cells, as indicated bellow by red arrows. MG1655 and MG1655.023, cells which are not colicinogenic, do not produce ZOI.
Characterization of the Anti-toxin Plasmid
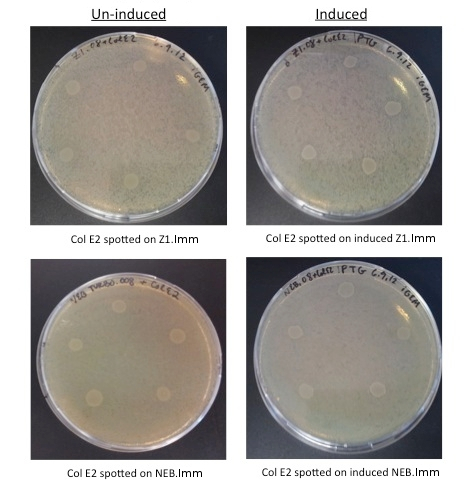
To test the function of our anti-toxin plasmid, we performed Colicin E2 toxicity assays on cells transformed with our plasmid. Here we expect zones of inhibition (ZOI, clear rings around the colicin cells) when Col E2 is spotted on vulnerable cells, and the absence of ZOI in the transformed immune cells.
Experimental setup
Cell Types
- MG1655: Wild Type Cells
- MG1655.023: MG1655 cells transformed with constitutive RFP (from Anderson promoter library, BBa_J61002)
- Col E2: Wild Type Colicin E2 cells, containing pColE2-P9 plasmid [2]
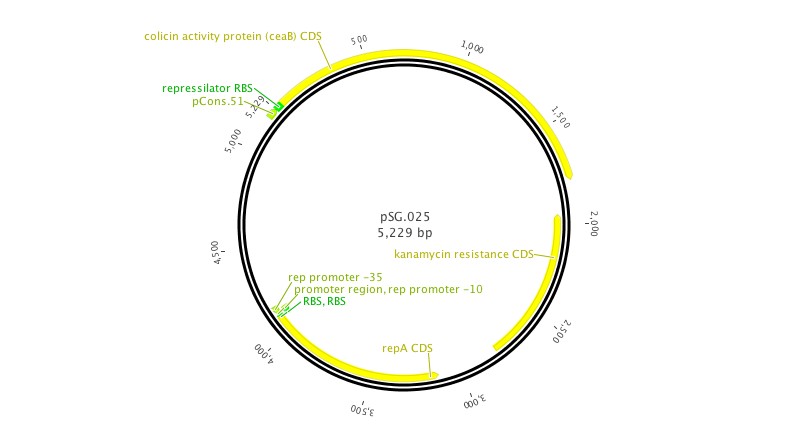
- NEB.008: NEB turbo cells transformed with the anti-toxin plasmid (pSG.008)
- ZI.008: MG1655 ZI cells transformed with the anti-toxin plasmid (pSG.008)
Protocol
- Grow cells in liquid culture containing the appropriate antibiotic
- Add IPTG (0.1mM) to appropriate liquid cultures after 2 hours (OD ~0.2)
- Wash cells containing antibiotics/IPTG and re-suspend in plain LB
- Plate 10 uL of 0.1 OD lawn cells from liquid culture on plain LB plates or IPTG plates
- Spot 5 uL of saturated Colicin E2 cells from liquid culture on plates
- Incubate plates overnight
Results
Col E2 cells do not generate a ZOI in cells containing the anti-toxin plasmid, in both induced and un-induced cells. However, Col E2 cells do produce a ZOI in vulnerable MG1655 cells as indicated by red arrows below.
Testing of the system
We want to characterize the immunity by varying the concentration of IPTG and the ColicinE2 cells.
Protocol
- Grow overnight culture pSG.001 (pSB3C5) and pSG.008 (pLac+Immunity) with antibiotic (Cm).
- Centrifuge and resuspend in LB.
- Prepare 5 tubes of 10ml LB and put 10uL of the cells into each tube.
- Incubate 37C until the OD reaches 0.2
- Put IPTG with different concentration in each tube (0.1mM, 0.05mM, 0.025mM, 0.0125mM and 0/no IPTG).
- Incubate again until the OD reaches 1.0
- Take each 0.5ml and mix with 0.5ml Colicin cells (saturated, saturated diluted 100x, and plain LB) in small 2ml eppendorf tubes.
- Incubate for 30 minutes.
- Resuspend in MgSO4 e-2M.
- Dilute 10000x (100x then 100x) in MgSO4 e-2M.
- Plate 20uL in Cm to kill the Colicin cells and leave the immune cells.
Note: 1ml of cells of OD 1.0 has 1e9 cells, so we expect if all cells survive we will get 1000 colonies at the end.
Results
Present your results
Related Parts and Links
- BBa_K131000 Colicin E2 operon, designed by Kevin McLeod, group: iGEM08_Calgary_Wetware (2008-07-22)
- BBa_K117001 Colicin E7 with immunity, designed by Nguyen Xuan Hung, group: iGEM08_NTU-Singapore (2008-10-07)
- BBa_K117000 Lysis gene (promotes lysis in colicin-producing bacteria strain), designed by Nguyen Xuan Hung, group: iGEM08_NTU-Singapore (2008-10-07)
- BBa_K117009 colicin E7 production system induced by lactose, designed by Nguyen Xuan Hung, group: iGEM08_NTU-Singapore (2008-10-08)
- BBa_R0040 TetR repressible promoter, designed by Designed by June Rhee, Connie Tao, Ty Thomson, Louis Waldman, group: Registry (2003-01-31)
- BBa_R0011 Promoter (lacI regulated, lambda pL hybrid), designed by Neelaksh Varshney, Grace Kenney, Daniel Shen, Samantha Sutton, group: Registry (2003-01-31)
- BBa_R0011:Experience/iGEM10 Kyoto pLac model
References
- Cascales E, et al. (2007) Colicin biology. Microbiol Mol Biol Rev 71:158–229.
- Majeed G, Gillor O, Kerr B, Riley MA. (2011) Competitive interactions in Escherichia coli populations: the role of bacteriocins. The ISME Journal 5, 71-81.
- Pugsley AP. (1985) Escherichia coli K12 strains for use in the identification and characterication of colicins. J Gen Microbiol 131: 369-376.
 "
"


 Overview
Overview Delay system
Delay system Semantic containment
Semantic containment Restriction enzyme system
Restriction enzyme system MAGE
MAGE Encapsulation
Encapsulation Synthetic import domain
Synthetic import domain Safety Questions
Safety Questions Safety Assessment
Safety Assessment