Team:Paris Bettencourt/Overview
From 2012.igem.org
| Line 3: | Line 3: | ||
<div id="grouptitle">Project Overview</div> | <div id="grouptitle">Project Overview</div> | ||
<br> | <br> | ||
| - | + | Explore our project by scrolling over the apps bellow! | |
<html> | <html> | ||
<style type="text/css"> | <style type="text/css"> | ||
Revision as of 02:28, 27 September 2012
Explore our project by scrolling over the apps bellow!





2) Semantic Containment: Our synthetic system will have an amber codon embedded in its genes. The amber suppressor system will ensure their expression in our bacteria, while preventing it in natural populations.

2) Semantic Containment: Our synthetic system will have an amber codon embedded in its genes. The amber suppressor system will ensure their expression in our bacteria, while preventing it in natural populations.
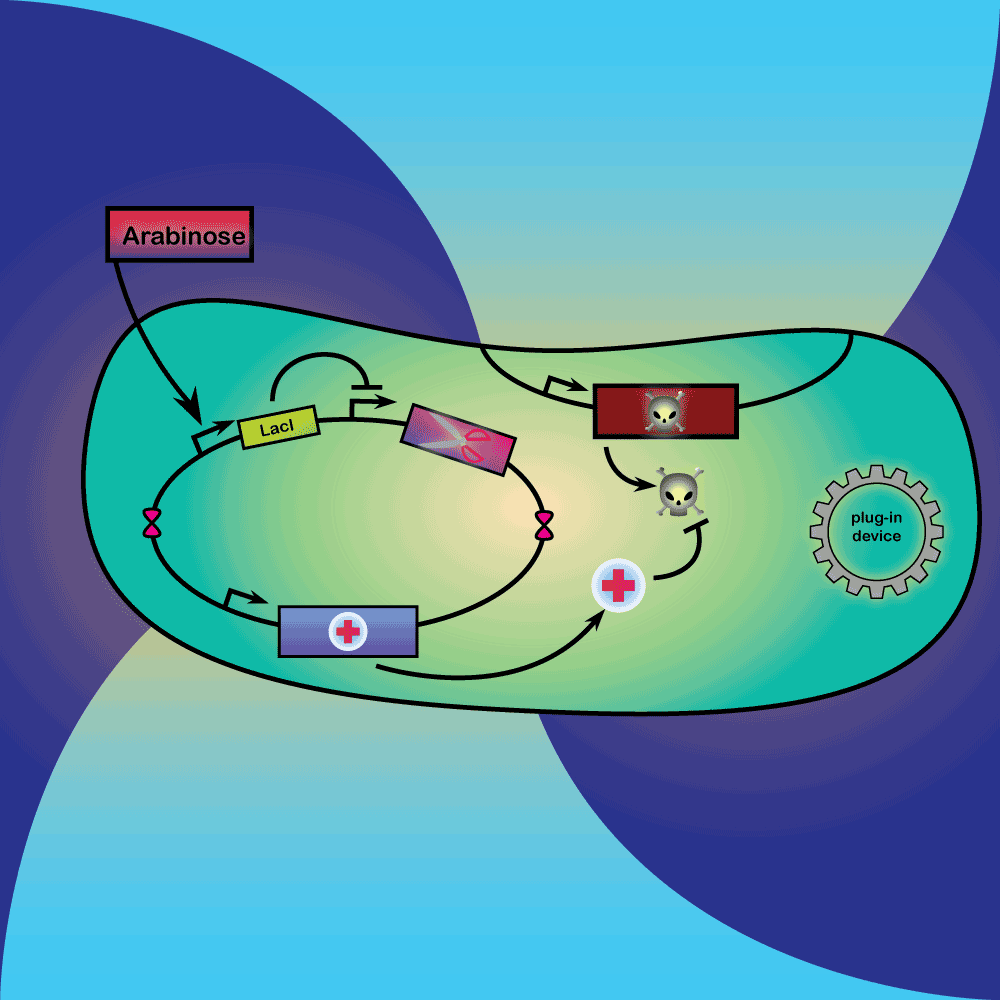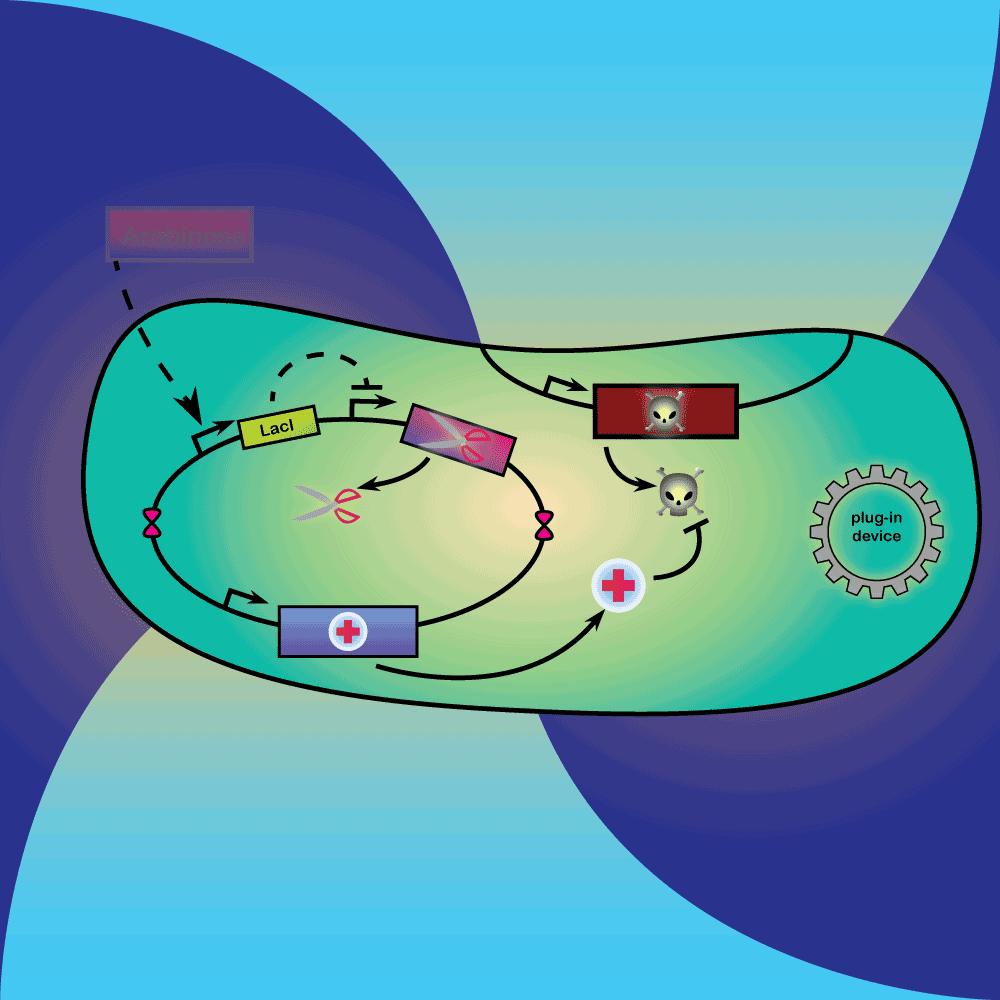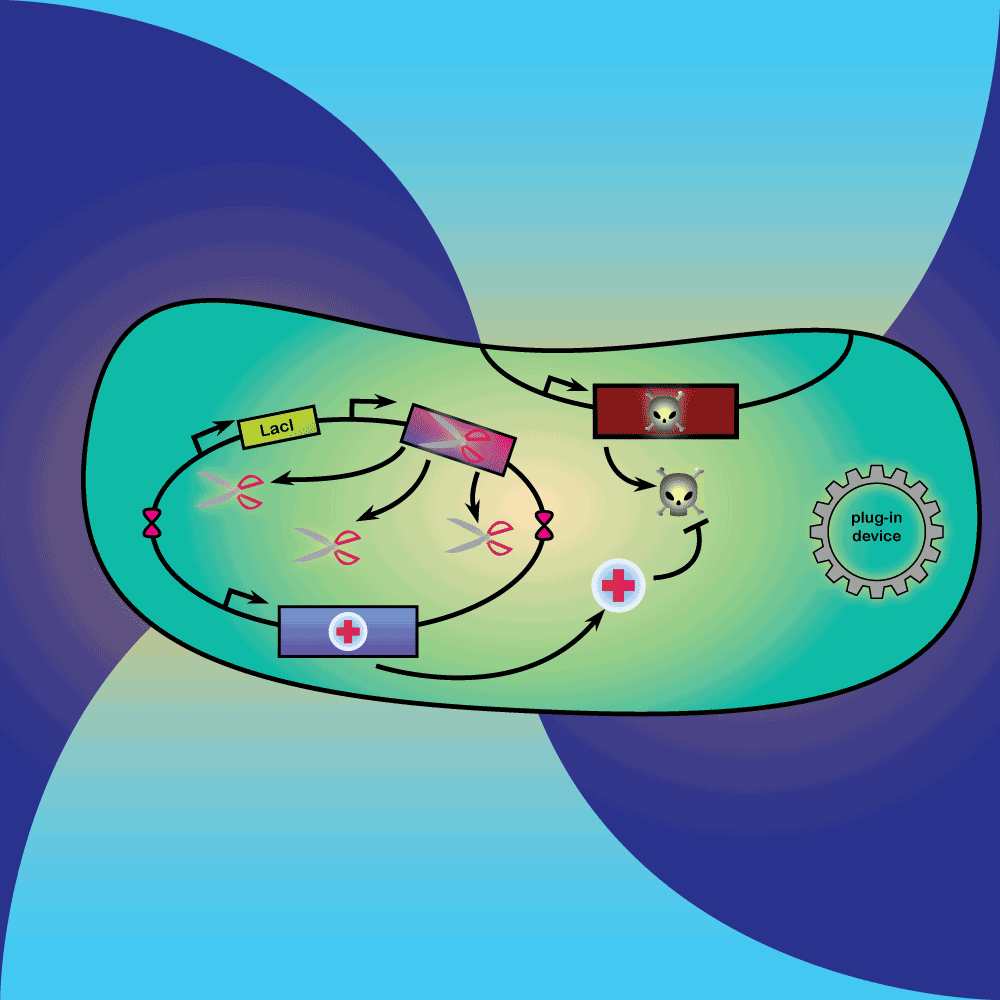
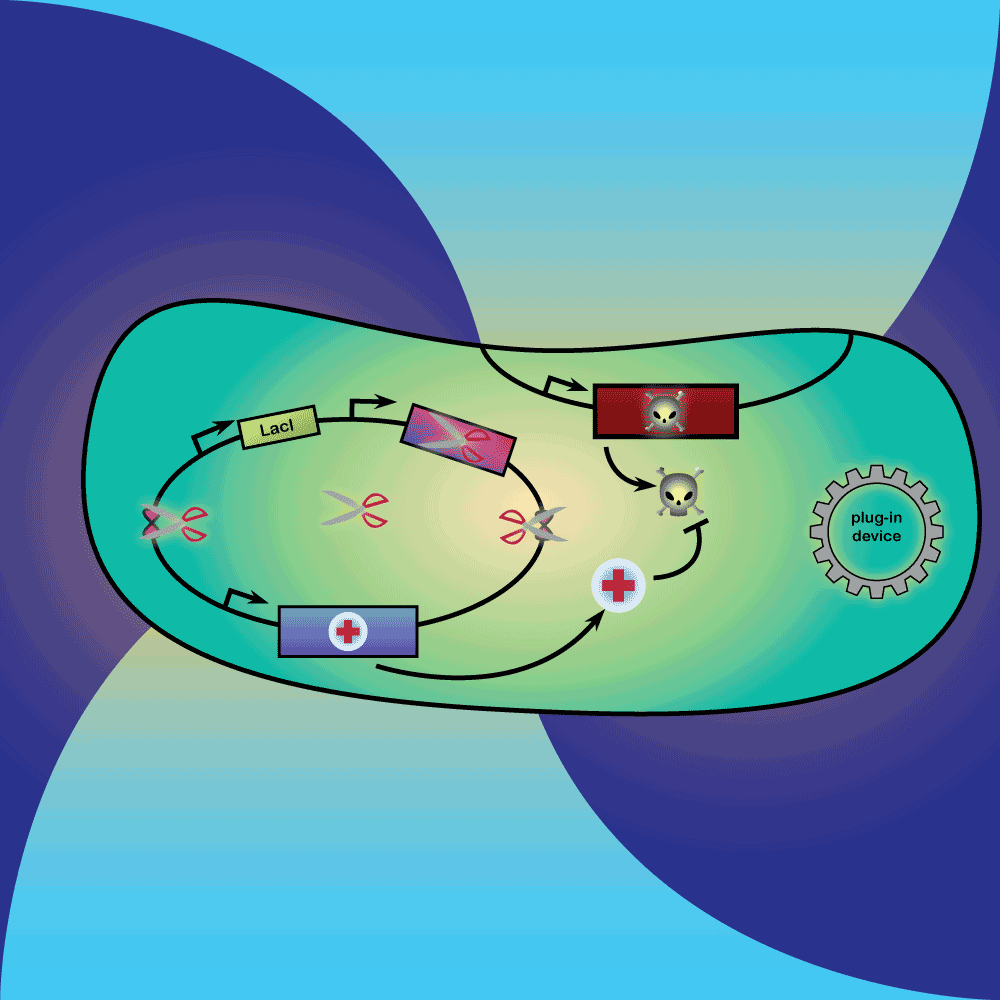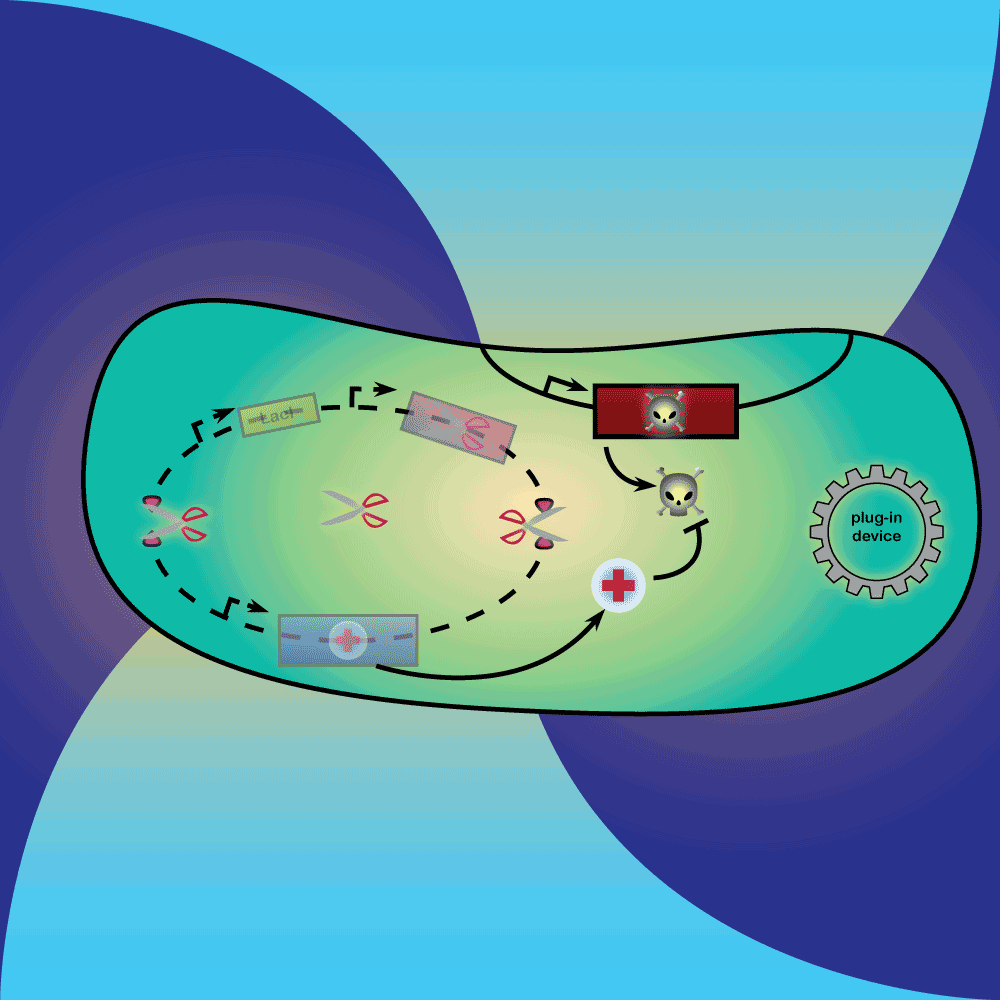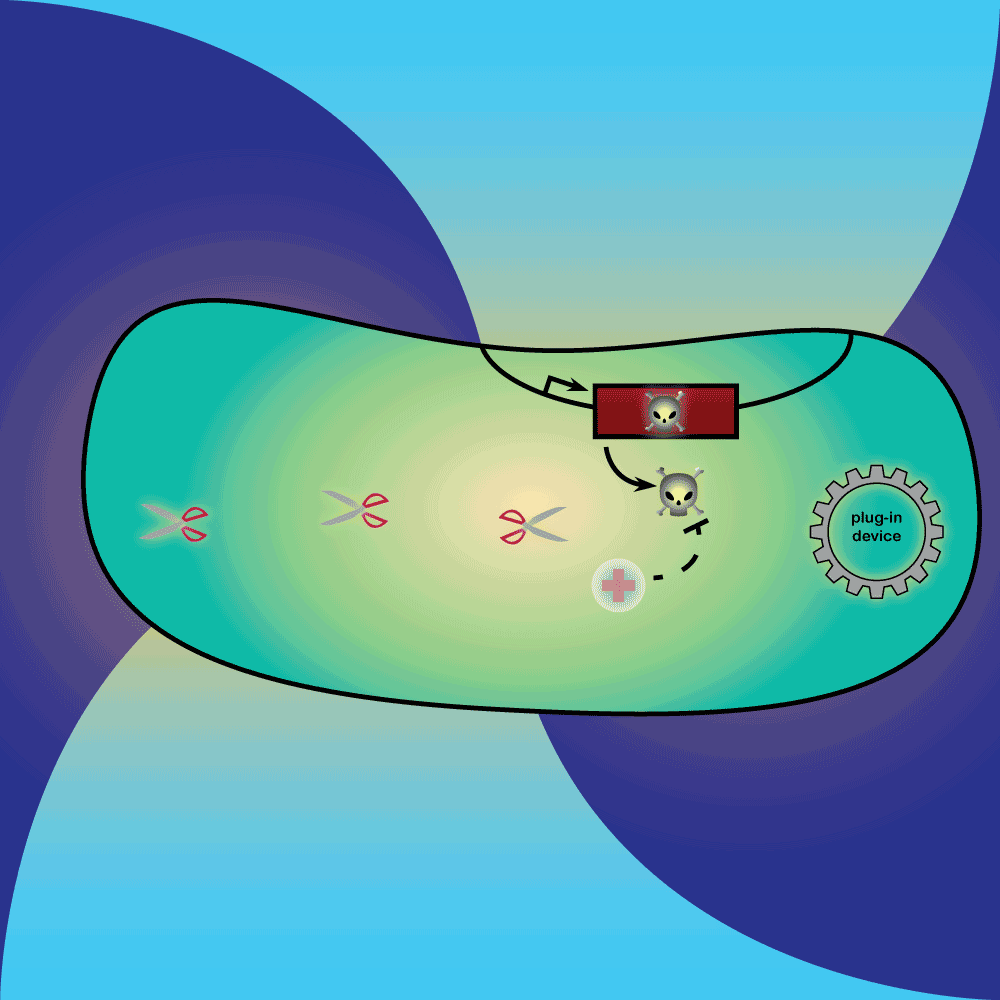
3) Delay system: In the presence of Arabinose, LacI is produced, repressing the expression of a restriction enzyme. Once Arabinose is not present any more, the LacI repressor concentration decreases with dilution and degradation. This leads to the expression of the restriction enzyme.

2) Semantic Containment: Our synthetic system will have an amber codon embedded in its genes. The amber suppressor system will ensure their expression in our bacteria, while preventing it in natural populations.
3) Delay system: In the presence of Arabinose, LacI is produced, repressing the expression of a restriction enzyme. Once Arabinose is not present any more, the LacI repressor concentration decreases with dilution and degradation. This leads to the expression of the restriction enzyme.
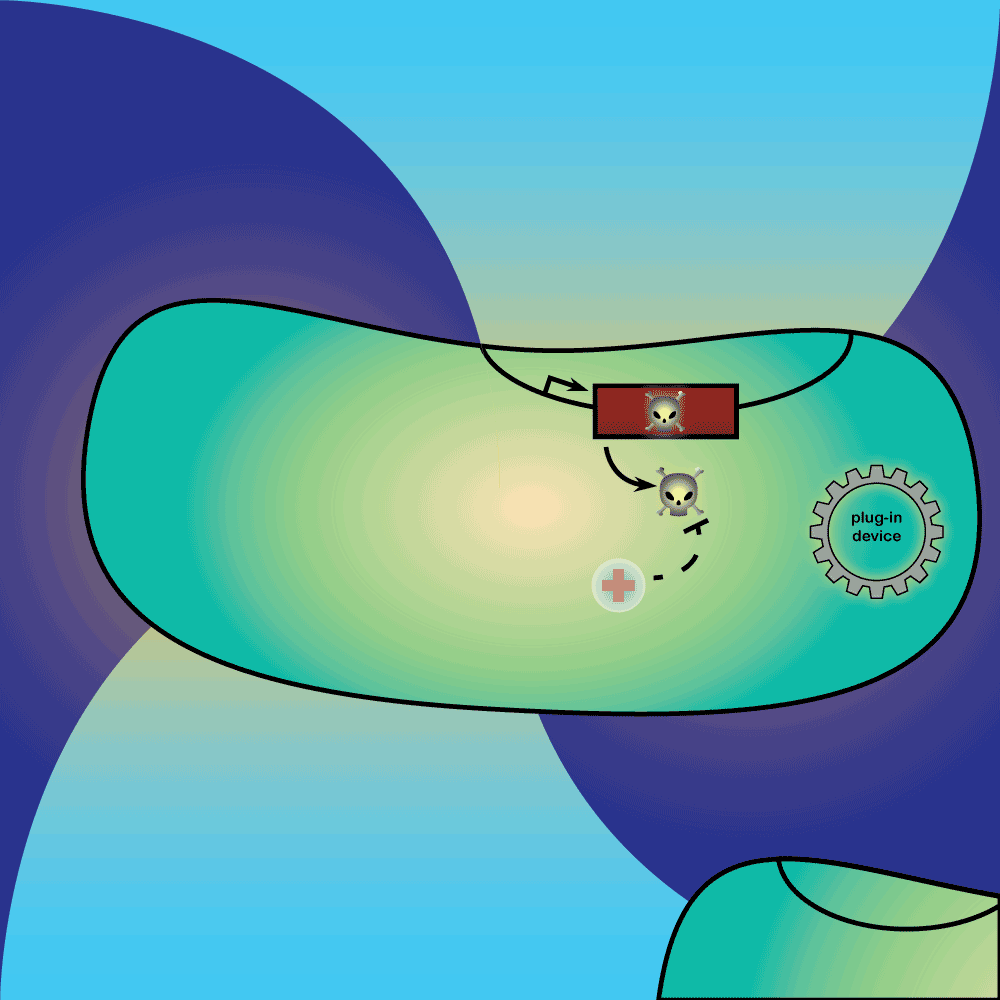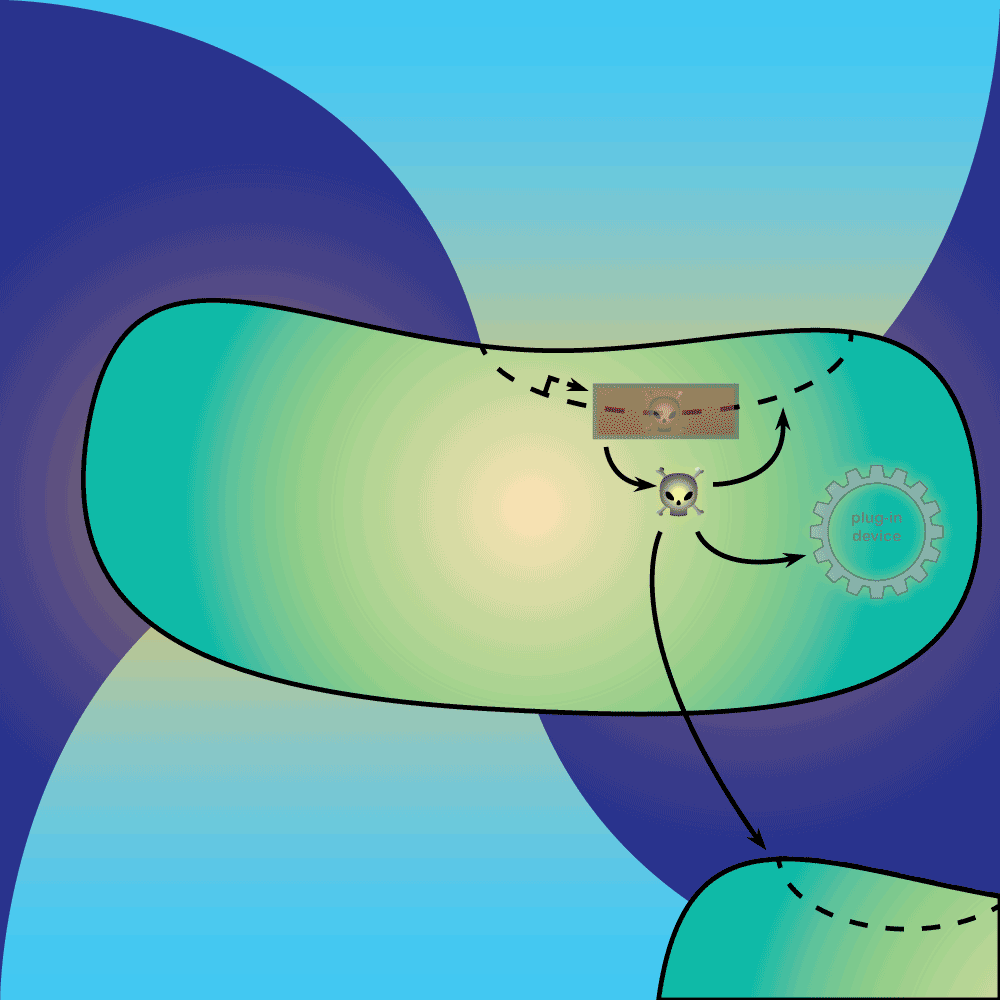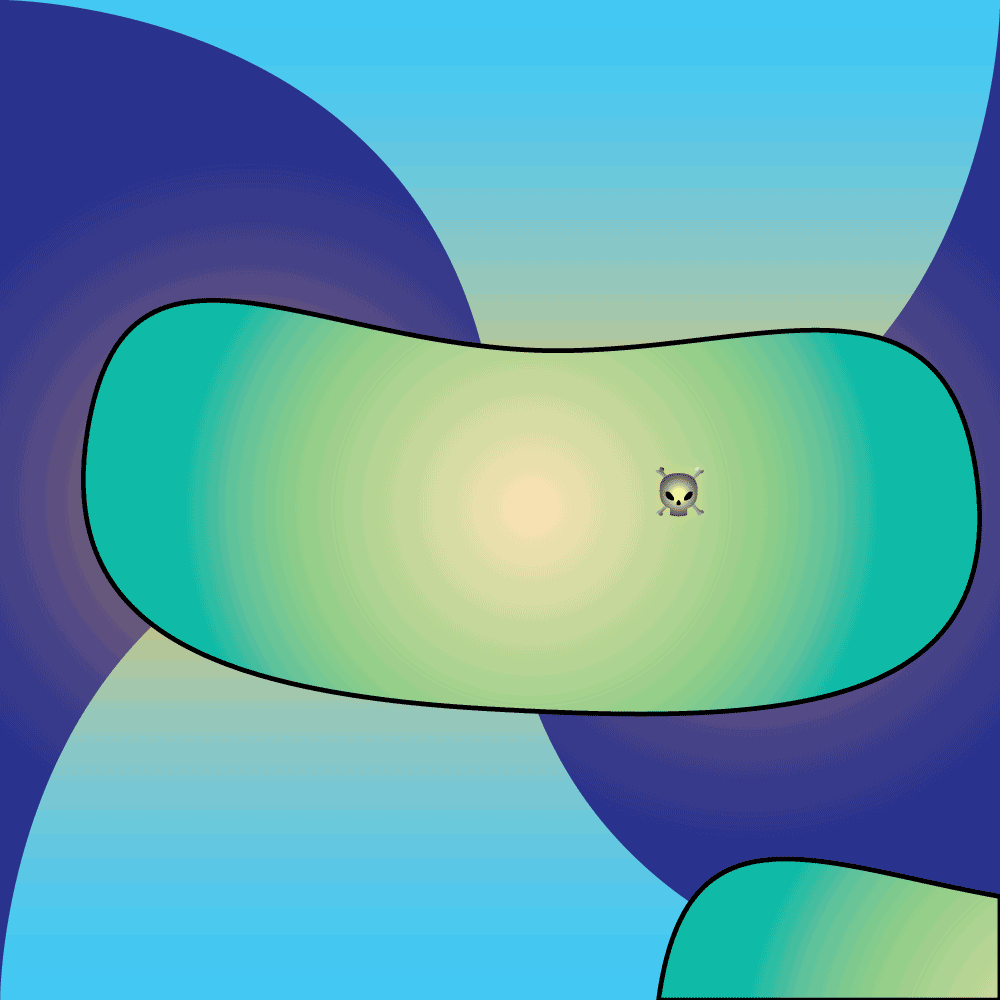
4) Restriction Enzyme system: The restriction enzyme destroys the plasmid carrying the synthetic circuit and the anti-toxin gene.

2) Semantic Containment: Our synthetic system will have an amber codon embedded in its genes. The amber suppressor system will ensure their expression in our bacteria, while preventing it in natural populations.
3) Delay system: In the presence of Arabinose, LacI is produced, repressing the expression of a restriction enzyme. Once Arabinose is not present any more, the LacI repressor concentration decreases with dilution and degradation. This leads to the expression of the restriction enzyme.
4) Restriction Enzyme system: The restriction enzyme destroys the plasmid carrying the synthetic circuit and the anti-toxin gene.
5) Suicide system: Once the anti-toxin concentration is below a given threshold, the toxin is no longer inhibited. It kills the cell as well as its neighbors, and eliminates extracellular DNA via its DNase activity.
Our project - a hypothetical case study
Imagine a farmer that wants measure the nutrient concentration in her field, the better to optimize her fertilizer use. We could provide her with cells carrying a nitrate biosensor (AgrEcoli), encapsulated in alginate beads. She would spread the beads in her field, wait for 12 hours, and then check if they are glowing in response to the nitrates in the soil.
We want this system to work the way the original designers intended. We also want to reduce the chance that the engineered bacteria will survive in the soil, release intact DNA, or transfer genes to a soil microbe.
Our system will be implemented as a pair of plasmids, compatible with the most commonly used BioBrick plasmids, and therefore easily integrated with existing systems. The AgrEColi are now running bWARE, and express a new containment functionality.
Shortly after the beads enter the soid, the delay system triggers and the DNA-degrading colicin proteins become active. The colicins specifically recognize and enter E. coli cells, killing them by completely degrading their genomes. The colicins also degrade DNA loose in the environment, without harming the native bacterial species.
Every field is a different ecosystem with a different composition of native species. The potential consequences of horizontal gene transfer are therefore difficult to predict in general. By destroying the information contained in DNA, our system reduces the chance that introduced DNA will replicate in the new environment.
Objectives
Our project aims to:
- Raise the issue of biosafety, and advocate the discerning use of biosafety circuits in future iGEM projects as a requirement
- Evaluate the risk of HGT in different SynBio applications
- Develop a new, improved containment system to expand the range of environments where GEOs can be used safely.
To do so, we:
- Engaged the general public and scientific community through debate
- Raised the question about how we can regulate this practices
- Compiled a parts page of safety circuits in the registry
- Relied on three levels of containment :
- Physical containment with alginate capsules
- Semantic containment using an amber suppressor system
- An improved killswitch featuring delayed population-level suicide through complete genome degradation.
We strived to make our system as robust against mutations as possible.
Key Killswitch Design Features
|
 "
"


 Overview
Overview Delay system
Delay system Restriction enzyme system
Restriction enzyme system MAGE
MAGE Synthetic import domain
Synthetic import domain Safety Questions
Safety Questions Safety Assessment
Safety Assessment