Team:Macquarie Australia/Protocols/GibsonTips
From 2012.igem.org
Top Ten Tips for Gibson Assembly
Gibson assembly is a powerful and useful tool for synthetic biologists. In our project, it was particularly useful for producing E. coli codon optimised BioBricks. In order to most effectively employ the technique of Gibson assembly, we designed DNA fragments known as G-blocks, which we assembled (as below) before combining and ligating them to produce our BioBricks.
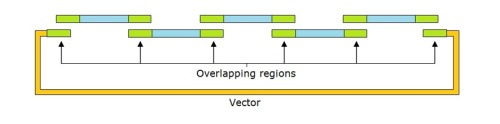
We found this process to be efficient and successful. We would recommend using the technique, but before you do- consider our top ten tips for designing and assembling G-blocks using Gibson assembly.
- Consider the overlapping sequence
The whole technique revolves around the overlapping sequence, everything can be planned but if this is not correct then the blocks won't anneal. Therefore, the overlapping regions need to be identified and conserved between the two adjacent sections G Blocks. Keep an eye on the Melting Temperature (TM), if this gets to high then significant problems will arise.
- Codon Usage
Optimising codons is one of the great things about Gibson Assembly, it allows us to increase the yield of our protein. Unfortunately, in our experience, optimising for E. Coli tends to increase the GC content considerably. As such a lot of the optimisation may need to be reversed to allow for the G Blocks to be synthesised.
- Be Wary of GC Rich Regions
As touched on in the previous point, GC rich regions make synthesis problematic. Hairpin loops are formed which prevent elongation. As such, when developing the G Blocks be wary of GC rich regions. If possible, using translation software, change bases in the wobble position to an A or T to keep the integrity of the protein sequence. We aimed to produce our gBlocks with less then 60% GC content.
- Check for Restriction Sites
With changes in the sequence following codon optimisation there is the risk that new restriction sites have been introduced. BioBricks require there to be no internalised EcoR1, Spe1, Xba1, or Pst1 sites. Therefore, the finals G Block sequence needs to be proofread for these sites or else the BioBrick is non-functional and so the Gibson Assembly becomes irrelevant.
- Be Meticulous
Like all molecular techniques, Gibson is very sensitive and so take care during the assembly. Ensure complete mixing and keep the enzymes being used on ice- otherwise they will denature and the reaction will not occur
- Use Electroporation for the transformation
You want to have the most efficient transformation or you will be doing several rounds of Gibson Assembly. In our project we found that electroporation was significantly more successful than heat shock.
- Use High Competency Cells
Gibson Assembly is a sensitive technique but only a small amount of complete plasmid is produced. To maximise to product obtained, highly efficient competent cells need to be used, or transformation procedures that provide higher efficiency need to be used.
Figure 1: the blue bars represent the electroporated transformation counts and the red represent the heat shock transformation counts. - Utilise the vector
When developing the gBlocks you can remove the need to perform restriction digests to produce a BioBrick. By taking the destination vector's 5' and 3' ends into account during development entire steps can be removed and the pure BioBrick can be easily produced. This is part of the power of Gibson Assembly and could be used to make complex plasmids.
- Beware of GC rich regions
No it is not a mistake, BEWARE THE GC RICH REGIONS.
- gBlock Provider
If the company you are ordering your gBlocks from is not in your time zone the development of the gBlocks may be slow. It is important that you develop good communication with one of their sales representatives. Communication is essential for this process, and poor communication can make a 4-day process significantly longer.
Our practical experience with Gibson assembly reassured us that this technique provides a successful method of gene cloning. Its high levels of efficiency convinced us that this procedure will quickly become the most widely used and accepted method of gene cloning in research and industry and will allow significant advances to be made in the field of synthetic biology.
 "
"





