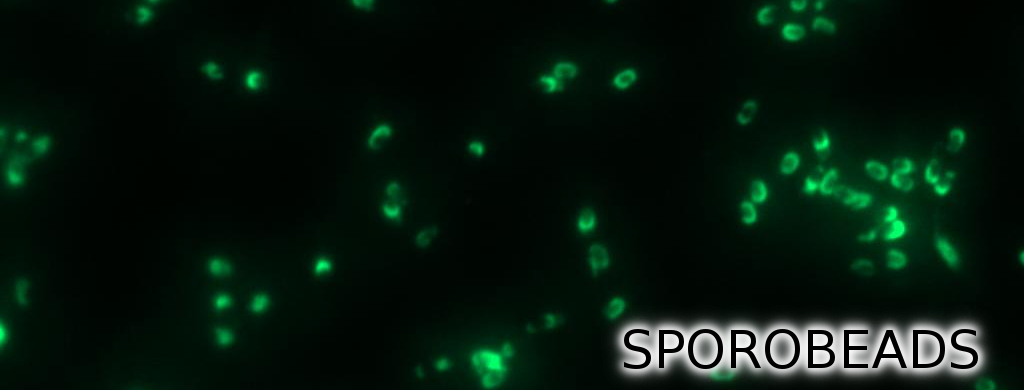
Team:LMU-Munich/Spore Coat Proteins/result
From 2012.igem.org
Franzi.Duerr (Talk | contribs) |
|||
| (19 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
| - | {{:Team:LMU-Munich/Templates/Page Header|File:Team- | + | {{:Team:LMU-Munich/Templates/Page Header|File:Team-LMU_eppis.resized.jpg|3}} |
| + | [[File:LMU Glow Spore2 cutII.jpg|620px]] | ||
| Line 6: | Line 7: | ||
| - | + | ====GFP as a Proof of Principle==== | |
| - | ==== | + | <br> |
| - | + | <p align="justify">Finally, we started with the most important experiment for our GFP-'''Sporo'''beads, the fluorescence microscopy. For a better understanding of our results, you can see in the table below the [https://2012.igem.org/Team:LMU-Munich/Spore_Coat_Proteins/cloning five constructs] and strain numbers we used in the following experiments. | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | <p align="justify"> | + | |
</p> | </p> | ||
| Line 50: | Line 46: | ||
| - | <p align="justify"> | + | <p align="justify">We developed a sporulation protocol (for details see [https://static.igem.org/mediawiki/2012/e/e9/LMU-Munich_2012_Protocol_for_enhancement_of_mature_spore_numbers.pdf Protocol for enhancement of mature spore numbers]) that increases the rates of mature spores in our samples. The cells were fixed on agarose pads and investigated by phase contrast and fluorescence microscopy. While spores of the wild type only showed the known background fluorescence, all '''Sporo'''beads showed bright green fluorescence at the edge (on the surface) of the spores. '''Sporo'''beads from strain B 53 (containing the P<sub>''cotYZ''</sub>-''cotZ''-2aa-''gfp''-terminator construct) showed the highest fluorescence intensity (see Fig. 5 and all [https://2012.igem.org/Team:LMU-Munich/Data/gfp_spore data]). Hence, this strain was chosen for further experiments.</p> |
| Line 67: | Line 63: | ||
| - | <p align="justify">Because of the low but distinct fluorescence of wild type spores, we measured and compared the fluorescence intensity of 100 spores per construct (see [https://2012.igem.org/Team:LMU-Munich/Data/gfp_spore data]). We obtained significant differences between wild type spores and all of our '''Sporo'''beads (see [https://2012.igem.org/Team:LMU-Munich/Data/gfp_spore data]). | + | This two short time lapse videos shows the formation of a spore and lollowing lysis of the mother cell. |
| - | The following graph (Fig. 6) shows the | + | |
| + | |||
| + | |||
| + | <html><div align="center"> | ||
| + | <iframe width="315" height="315" src="http://www.youtube.com/embed/fXhUsB4dTIs" frameborder="0" allowfullscreen></iframe></div> | ||
| + | </html> | ||
| + | |||
| + | <br> | ||
| + | <br> | ||
| + | <p align="justify">Because of the low but distinct fluorescence of wild type spores, we measured and compared the fluorescence intensity of 100 spores per construct (see [https://2012.igem.org/Team:LMU-Munich/Data/gfp_spore data]). We obtained significant differences between wild type spores and all of our '''Sporo'''beads (see [https://2012.igem.org/Team:LMU-Munich/Data/gfp_spore data]). The following graph (Fig. 6) shows the microscopy pictures and analysis of the strongest of the five constructs integrated into wildtype W168 (B53) and the deletion strain B 49 (B70).</p> | ||
| Line 80: | Line 85: | ||
{| style="color:black;" cellpadding="0" width="100%" cellspacing="0" border="0" align="center" style="text-align:center;" | {| style="color:black;" cellpadding="0" width="100%" cellspacing="0" border="0" align="center" style="text-align:center;" | ||
|style="width: 70%;background-color: #EBFCE4;" | | |style="width: 70%;background-color: #EBFCE4;" | | ||
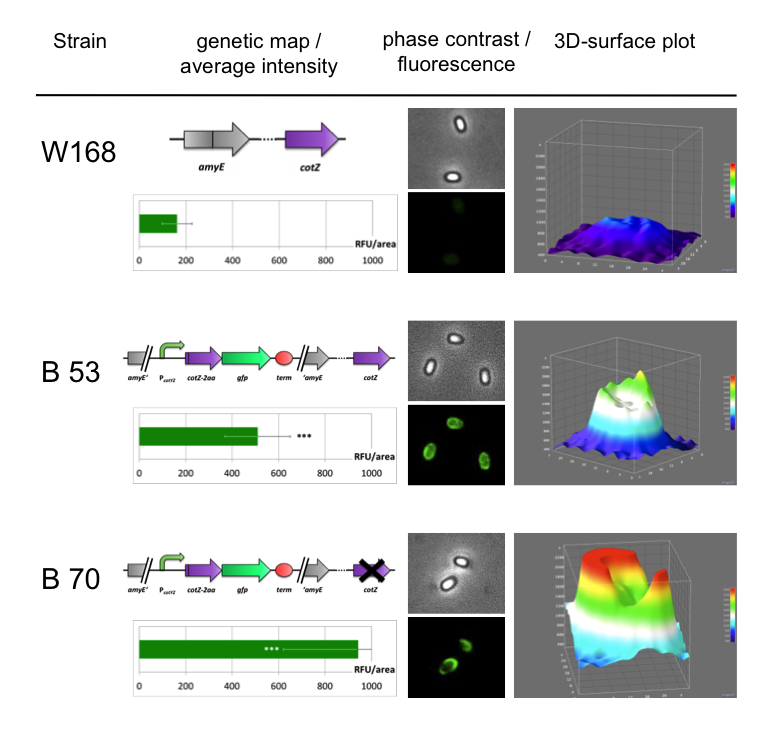
| - | <font color="#000000"; size="2">Fig. 6: Result of fluorescence evaluation of the three strains W168, B53 and B70.</font> | + | <font color="#000000"; size="2">Fig. 6: Result of fluorescence evaluation of the three strains W168, B53 and B70. The bar charts show the average fluorescence intensity over 100-200 spores. For image analysis we measured the fluorescence intensity of an area of 750 pixel per spore by using ImageJ and evaluated the results with the statistical software R. The 3D graphs illustrate in one spore the distribution of fluorescence intensity across the spore surface.</font> |
|} | |} | ||
|} | |} | ||
| Line 87: | Line 92: | ||
<p align="justify">As shown in Fig. 6, the wild type spore has hardly any fluorescence, whereas both''' Sporo'''beads with the integrated construct pSB<sub>''Bs''</sub>1C-P<sub>''cotYZ''</sub>-''cotZ''<sub>-2aa</sub>-''gfp''-terminator give a distinct fluorescence signal around the edge of the spore. Furthermore, it demonstrates that strain B 70 has the highest fluorescence intensity. For more detailed information look at our [https://2012.igem.org/Team:LMU-Munich/Data/gfp_spore Data page]</p> | <p align="justify">As shown in Fig. 6, the wild type spore has hardly any fluorescence, whereas both''' Sporo'''beads with the integrated construct pSB<sub>''Bs''</sub>1C-P<sub>''cotYZ''</sub>-''cotZ''<sub>-2aa</sub>-''gfp''-terminator give a distinct fluorescence signal around the edge of the spore. Furthermore, it demonstrates that strain B 70 has the highest fluorescence intensity. For more detailed information look at our [https://2012.igem.org/Team:LMU-Munich/Data/gfp_spore Data page]</p> | ||
| - | <p align="justify">In summary we successfully developed functional | + | <p align="justify">In summary we successfully developed functional '''Sporo'''beads that are capable of displaying any protein of choice on the surface of modified ''B. subtilis'' endospores.</p> |
| Line 95: | Line 100: | ||
| - | [[File: | + | {| width="100%" cellpadding="20" |
| - | + | |[[File:LMU Arrow purple BACK.png|right|80px|link=Team:LMU-Munich/Spore_Coat_Proteins]] | |
| + | |[[File:LMU Arrow purple NEXT.png|left|80px|link=Team:LMU-Munich/Spore_Coat_Proteins/purification]] | ||
| + | |} | ||
{{:Team:LMU-Munich/Templates/Page Footer}} | {{:Team:LMU-Munich/Templates/Page Footer}} | ||
Latest revision as of 17:02, 26 October 2012

The LMU-Munich team is exuberantly happy about the great success at the World Championship Jamboree in Boston. Our project Beadzillus finished 4th and won the prize for the "Best Wiki" (with Slovenia) and "Best New Application Project".
[ more news ]

GFP as a Proof of Principle
Finally, we started with the most important experiment for our GFP-Sporobeads, the fluorescence microscopy. For a better understanding of our results, you can see in the table below the five constructs and strain numbers we used in the following experiments.
| recipient strain W168 | recipient strain B 49 (W168 ΔcotZ) | |
|---|---|---|
| pSBBs1C-PcotYZ-cotZ-2aa-gfp-terminator | B 53 | B 70 |
| pSBBs1C-PcotYZ-cotZ-gfp-terminator | B 54 | B 71 |
| pSBBs1C-PcotV-cotZ-2aa-terminator | B 55 | B 72 |
| pSBBs1C-PcotV-cotZ-terminator | B 56 | B 73 |
| pSBBs1C-PcgeA-cotZ-2aa-terminator | B 52 | B 69 |
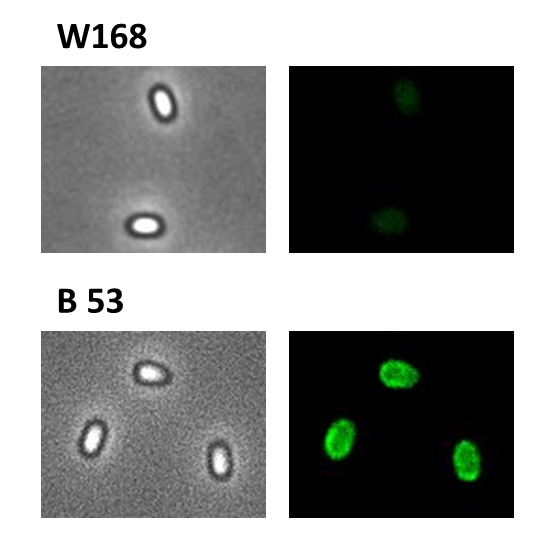
We developed a sporulation protocol (for details see Protocol for enhancement of mature spore numbers) that increases the rates of mature spores in our samples. The cells were fixed on agarose pads and investigated by phase contrast and fluorescence microscopy. While spores of the wild type only showed the known background fluorescence, all Sporobeads showed bright green fluorescence at the edge (on the surface) of the spores. Sporobeads from strain B 53 (containing the PcotYZ-cotZ-2aa-gfp-terminator construct) showed the highest fluorescence intensity (see Fig. 5 and all data). Hence, this strain was chosen for further experiments.
|
This two short time lapse videos shows the formation of a spore and lollowing lysis of the mother cell.
Because of the low but distinct fluorescence of wild type spores, we measured and compared the fluorescence intensity of 100 spores per construct (see data). We obtained significant differences between wild type spores and all of our Sporobeads (see data). The following graph (Fig. 6) shows the microscopy pictures and analysis of the strongest of the five constructs integrated into wildtype W168 (B53) and the deletion strain B 49 (B70).
|
| |
|
As shown in Fig. 6, the wild type spore has hardly any fluorescence, whereas both Sporobeads with the integrated construct pSBBs1C-PcotYZ-cotZ-2aa-gfp-terminator give a distinct fluorescence signal around the edge of the spore. Furthermore, it demonstrates that strain B 70 has the highest fluorescence intensity. For more detailed information look at our Data page
In summary we successfully developed functional Sporobeads that are capable of displaying any protein of choice on the surface of modified B. subtilis endospores.
 "
"