|
|
| (28 intermediate revisions not shown) |
| Line 1: |
Line 1: |
| | <!-- Include the next line at the beginning of every page --> | | <!-- Include the next line at the beginning of every page --> |
| | {{:Team:LMU-Munich/Templates/Page Header|File:Team-LMU_eppis.resized.jpg|3}} | | {{:Team:LMU-Munich/Templates/Page Header|File:Team-LMU_eppis.resized.jpg|3}} |
| | + | [[File:LMU Glow Spore2 cutII.jpg|620px|link=]] |
| | | | |
| | | | |
| - | [[File:LMU Glow Spore2 cutII.jpg|620px]] | + | [[File:SporeCoat.png|100px|right|link=]] |
| | | | |
| - | [[File:SporeCoat.png|100px|right|link=Team:LMU-Munich/Spore_Coat_Proteins]]
| |
| | | | |
| | | | |
| | + | =='''Sporo'''beads - What Protein Do ''You'' Want to Display?== |
| | + | <br> |
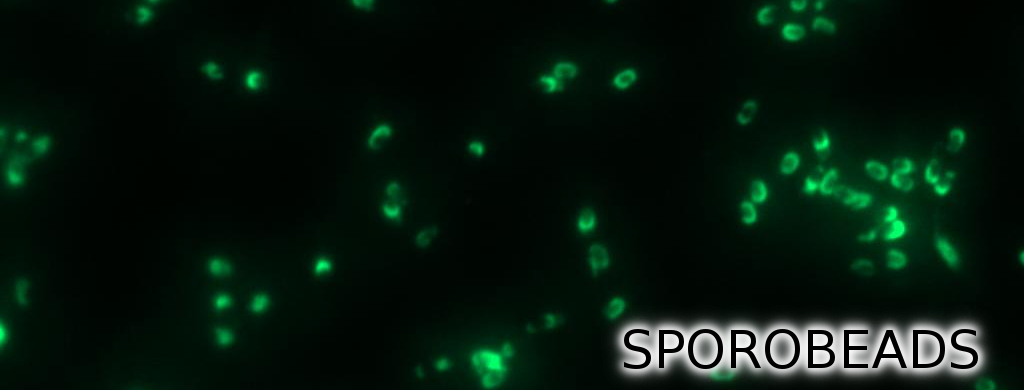
| | + | <p align="justify">'''Sporo'''beads are the first generation of ''Bacillus subtilis'' endospores displaying a protein of our choice on their outermost layer, the spore crust. In future '''Sporo'''beads could serve as a platform for protein display and thus be used for numerous versatile [https://2012.igem.org/Team:LMU-Munich/Application applications]. Our goal was to show that ''B. subtilis'' spores have the ability to do so. As a [https://2012.igem.org/Team:LMU-Munich/Spore_Coat_Proteins/result proof of principle] we successfully fused GFP to the spore crust and obtained fluorescence with microscopy.</p> |
| | | | |
| | | | |
| - | =='''Sporo'''beads - what protein do ''you'' want to display?==
| |
| - | <br>
| |
| | <div class="box"> | | <div class="box"> |
| - | ===What are '''Sporo'''beads?=== | + | ===Scientific Background=== |
| | {| "width=100%" style="text-align:center;" style="align:right"| | | {| "width=100%" style="text-align:center;" style="align:right"| |
| - | |<p align="justify">Introduction to ''B. subtilis'' spores and their assignment for our project</p> | + | |<p align="justify">Introduction to ''B. subtilis'' spores and their use in our project</p> |
| | |[[File:Imamura, 2011 & McKenney, 2010.png|200px|right|link=Team:LMU-Munich/Spore_Coat_Proteins/Background]] | | |[[File:Imamura, 2011 & McKenney, 2010.png|200px|right|link=Team:LMU-Munich/Spore_Coat_Proteins/Background]] |
| | |- | | |- |
| Line 23: |
Line 24: |
| | | | |
| | <div class="box"> | | <div class="box"> |
| - | ===Crust Promoter Evaluation=== | + | ===Cloning Strategy=== |
| - | {| "width=100%" style="text-align:center;"| | + | {| "width=100%" style="text-align:center;" style="align:right"| |
| - | |<p align="justify">Three sporulation-dependent promoters (P<sub>''cotYZ''</sub>, P<sub>''cotV''</sub>, P<sub>''cgeA''</sub>) were constructed, two of which show the expected behavior.</p> | + | |<p align="justify">Cloning strategy to create different variants of our ''' Sporo'''beads</p> |
| - | |[[File:Promotoren.png|200px|link=Team:LMU-Munich/Data/crustpromoters]] | + | |[[File:Final construct.png|200px|link=Team:LMU-Munich/Spore_Coat_Proteins/cloning]] |
| | |- | | |- |
| - | ! colspan="2" |[[File:LMU Arrow purple.png|40px|link=Team:LMU-Munich/Data/crustpromoters]] | + | ! colspan="2" |[[File:LMU Arrow purple.png|40px|link=Team:LMU-Munich/Spore_Coat_Proteins/cloning]] |
| | |} | | |} |
| | </div> | | </div> |
| | | | |
| | <div class="box"> | | <div class="box"> |
| - | ===Crust protein gene organization=== | + | ===GFP as a Proof of Principle=== |
| | {| "width=100%" style="text-align:center;" style="align:right"| | | {| "width=100%" style="text-align:center;" style="align:right"| |
| - | |<p align="justify">Location of the two crust protein genes in the genome</p> | + | |<p align="justify">Main results of the various constructs that were created to find the best one!</p> |
| - | |[[File:Operons.png|200px|link=Team:LMU-Munich/Spore_Coat_Proteins/crustproteins]] | + | |[[File:LMU Firstspore.jpg|200px|right|link=Team:LMU-Munich/Spore_Coat_Proteins/result]] |
| | |- | | |- |
| - | ! colspan="2" |[[File:LMU Arrow purple.png|40px|link=Team:LMU-Munich/Spore_Coat_Proteins/crustproteins]] | + | ! colspan="2" |[[File:LMU Arrow purple.png|40px|link=Team:LMU-Munich/Spore_Coat_Proteins/result]] |
| | |} | | |} |
| | </div> | | </div> |
| | | | |
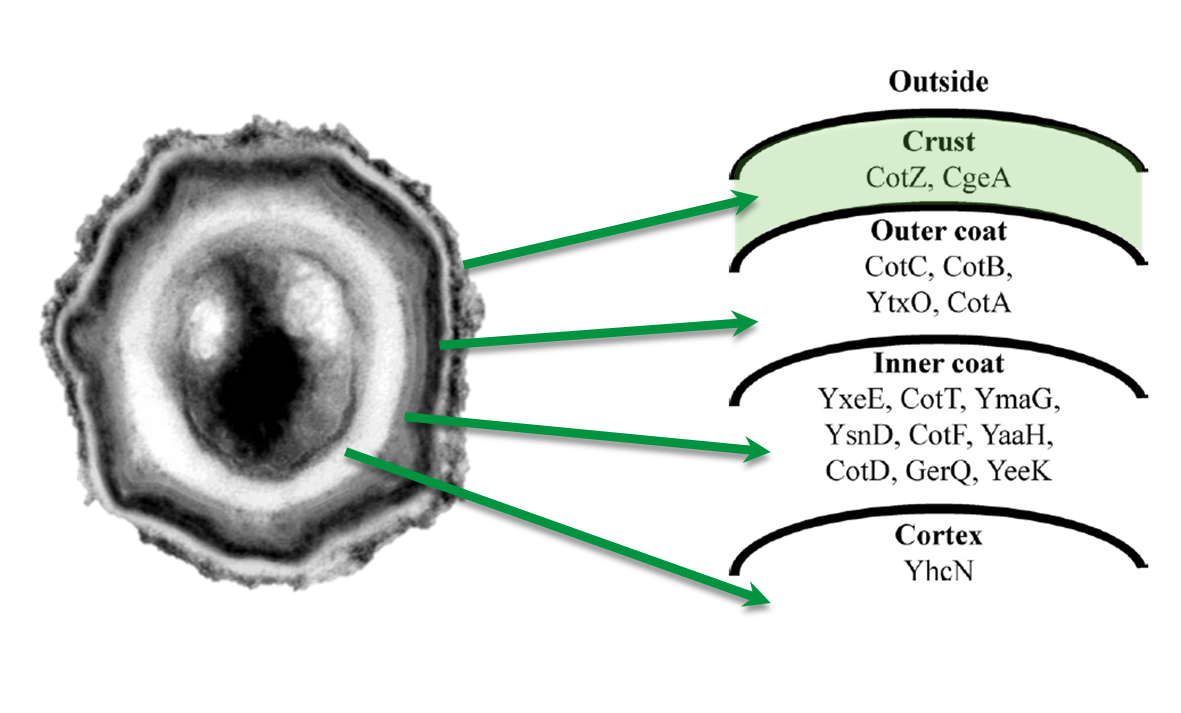
| - | <p align="justify">The gene ''cgeA'' is located in the ''cgeABCDE'' cluster and is expressed from its own promoter P<sub>''cgeA''</sub>. The cluster ''cotVWXYZ'' contains the gene ''cotZ'' which is cotranscribed with ''cotY'' and expressed from the promoter P<sub>''cotYZ''</sub>. Another promoter of this cluster, P<sub>''cotV''</sub>, is responsible for the transcription of the other three genes (Fig. 2). Those three promoters were evaluated with the ''lux'' reporter genes ([http://partsregistry.org/Part:BBa_K823025 pSB<sub>''Bs''</sub>3C-''lux''ABCDE]) to analyze their strength and the time point of their activation (see [https://2012.igem.org/Team:LMU-Munich/Data/crustpromoters data]).</p> | + | <div class="box"> |
| - | | + | ===Laccases as functional enzymes=== |
| - | | + | {| "width=100%" style="text-align:center;" style="align:right"| |
| - | {| style="color:black;" cellpadding="3" width="100%" cellspacing="0" border="0" align="center" style="text-align:left;" | + | |<p align="justify">Creation of functional Laccase-''' Sporo'''beads</p> |
| - | | style="width: 100%;background-color: #EBFCE4;" |
| + | |[[File:Final construct.png|200px|link=Team:LMU-Munich/Spore_Coat_Proteins/laccases]] |
| - | {|
| + | |
| - | |[[File:Operons.png|610px|center]] | + | |
| | |- | | |- |
| - | | style="width: 70%;background-color: #EBFCE4;" |
| + | ! colspan="2" |[[File:LMU Arrow purple.png|40px|link=Team:LMU-Munich/Spore_Coat_Proteins/laccases]] |
| - | {| style="color:black;" cellpadding="0" width="70%" cellspacing="0" border="0" align="center" style="text-align:center;"
| + | |
| - | |style="width: 70%;background-color: #EBFCE4;" | | + | |
| - | <font color="#000000"; size="2">Fig. 2: Gene clusters of ''cotZ'' and ''cgeA''</font>
| + | |
| - | |}
| + | |
| - | |}
| + | |
| | |} | | |} |
| | + | </div> |
| | | | |
| - | | + | <div class="box"> |
| - | <p align="justify">Based on this data, two of the three promoters could be used for expression of spore crust fusion proteins (Fig. 3). First, we used [http://partsregistry.org/Part:BBa_K823039 ''gfp''] as a proof of principle and fused it to ''cgeA'' and [http://partsregistry.org/wiki/index.php?title=Part:BBa_K823031 ''cotZ'']. This way, we can determine if it is possible to display proteins on the spore crust and if their expression has any effect on spore formation.</p> | + | ===Purification Methods=== |
| - | | + | {| "width=100%" style="text-align:center;" style="align:right"| |
| - | | + | |<p align="justify">Description of the different purification methods of the spores</p> |
| - | [[File:Spore crust proteins cycle.jpg|600px|right]]
| + | |[[File:Treatments.png|200px|right|link=Team:LMU-Munich/Spore_Coat_Proteins/purification]] |
| - | {| style="color:black;" cellpadding="3" width="100%" cellspacing="0" border="0" align="center" style="text-align:center;" | + | |
| - | | style="width: 100%;background-color: #FFFFFF;" | | + | |
| | |- | | |- |
| - | | style="width: 70%;background-color: #FFFFFF;" |
| + | ! colspan="2" |[[File:LMU Arrow purple.png|40px|link=Team:LMU-Munich/Spore_Coat_Proteins/purification]] |
| - | {| style="color:black;" cellpadding="0" width="70%" cellspacing="0" border="0" align="center" style="text-align:center;"
| + | |
| - | |style="width: 70%;background-color: #FFFFFF;" | | + | |
| - | <font color="#000000"; size="2">Fig. 3: The '''Bead'''zillus cycle</font>
| + | |
| | |} | | |} |
| - | |}
| + | </div> |
| - |
| + | <br> |
| - | | + | <br> |
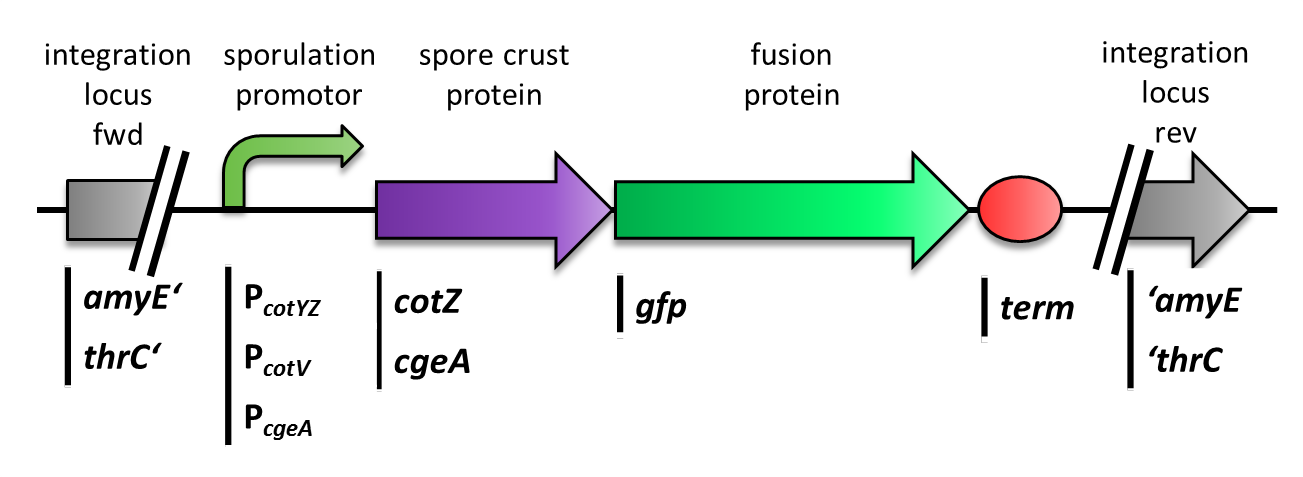
| - | <p align="justify">We constructed the BioBricks for ''cotZ'', ''cgeA'' and ''gfp'' in [http://partsregistry.org/Help:Assembly_standard_25 Freiburg Standard]. The ''cotZ'' gene was then fused to its two native promoters, P<sub>''cotV''</sub> and to P<sub>''cotYZ''</sub>, and P<sub>''cgeA''</sub>, which regulates the transcription of ''cgeA''. For ''cgeA'' we only used its native promoter P<sub>''cgeA''</sub> and the stronger one of the two promoters of the ''cotVWXYZ'' cluster, P<sub>''cotYZ''</sub> (for more details see [https://2012.igem.org/Team:LMU-Munich/Data/crustpromoters crust promotor evaluation]). In addition [http://partsregistry.org/Part:BBa_K823039 ''gfp''] was ligated to the [http://partsregistry.org/wiki/index.php?title=Part:BBa_B0014 terminator B0014]. After sequence confirmation, we fused the ''cgeA''/''cotZ''- and ''gfp''-constructs together, applying the [http://partsregistry.org/Help:Assembly_standard_25 Freiburg standard] to create in-frame fusion proteins. This way, we created C-terminal ''gfp'' fusion to both spore crust proteins flanked by the promoters and terminator above (Fig. 4). </p> | + | <br> |
| - | | + | <br> |
| - | | + | |
| - | | + | |
| - | {| style="color:black;" cellpadding="3" width="100%" cellspacing="0" border="0" align="center" style="text-align:left;"
| + | |
| - | | style="width: 70%;background-color: #EBFCE4;" |
| + | |
| - | {|
| + | |
| - | |[[File:Final construct.png|610px|center]]
| + | |
| - | |}
| + | |
| - | |-
| + | |
| - | | style="width: 70%;background-color: #EBFCE4;" |
| + | |
| - | {| style="color:black;" cellpadding="0" width="100%" cellspacing="0" border="0" align="center" style="text-align:center;"
| + | |
| - | |style="width: 70%;background-color: #EBFCE4;" |
| + | |
| - | <font color="#000000"; size="2">Fig. 4: Section of the genome of ''B. subtilis'' with the various integrated constructs.</font> | + | |
| - | |}
| + | |
| - | |}
| + | |
| - | | + | |
| - | | + | |
| - | <p align="justify"><br>But as we did not know if C- or N-terminal fusion would influence the fusion protein expression, our second aim was to construct N-terminal fusion proteins as well. For this purpose we wanted to fuse the genes for the crust proteins ''cotZ'' and ''cgeA'' to the terminator and ''gfp'' to the three chosen promoters. Unfortunately, there occured a mutation in the XbaI site during construction of ''gfp'' in Freiburg Standard. Therefore we were not able to finish these constructs. Finally we needed to clone our constructs into an empty ''Bacillus'' vector, so that they could get integrated into the genome of ''B. subtilis'' after transformation. For that purpose, we picked the empty vector pSB<sub>BS</sub>1C from our [https://2012.igem.org/Team:LMU-Munich/Bacillus_BioBricks#Bacillus_Vectors '''''Bacillus''B'''io'''B'''rick'''B'''ox], for the ''cotZ'' constructs. This vector integrates into the ''amyE'' locus, which allows to easily check the integration by starch test. In order to also express both crust protein constructs in one strain, the ''cgeA'' fusion proteins had to be cloned into another empty vector, pSB<sub>BS</sub>4S. Unfortunately, for unknown reasons, the cloning of the constructs with ''cgeA'' into this vector has so far not been successful.
| + | |
| | <br> | | <br> |
| - | <br>But we were able to finish five constructs and integrated them into wild type W168 and the Δ''cotZ'' mutant:
| |
| - | </p>
| |
| - |
| |
| - |
| |
| - | {| class="colored" width="100%" align="center" style="text-align:center;"
| |
| - | |-
| |
| - | |style="width:52%;"|
| |
| - | |style="width:20%;"|
| |
| - | |style="width:28%;"|
| |
| - | |-
| |
| - | !
| |
| - | !recipient strain W168
| |
| - | !recipient strain B 49 (W168 Δ''cotZ'')
| |
| - | |-
| |
| - | !pSB<sub>''Bs''</sub>1C-P<sub>''cotYZ''</sub>-''cotZ''-2aa-''gfp''-terminator
| |
| - | |B 53
| |
| - | |B 70
| |
| - | |-
| |
| - | !pSB<sub>''Bs''</sub>1C-P<sub>''cotYZ''</sub>-''cotZ''-''gfp''-terminator
| |
| - | |B 54
| |
| - | |B 71
| |
| - | |-
| |
| - | !pSB<sub>''Bs''</sub>1C-P<sub>''cotV''</sub>-''cotZ''-2aa-terminator
| |
| - | |B 55
| |
| - | |B 72
| |
| - | |-
| |
| - | ! pSB<sub>''Bs''</sub>1C-P<sub>''cotV''</sub>-''cotZ''-terminator
| |
| - | |B 56
| |
| - | |B 73
| |
| - | |-
| |
| - | !pSB<sub>''Bs''</sub>1C-P<sub>''cgeA''</sub>-''cotZ''-2aa-terminator
| |
| - | |B 52
| |
| - | |B 69
| |
| - | |-
| |
| - | |}
| |
| - |
| |
| - |
| |
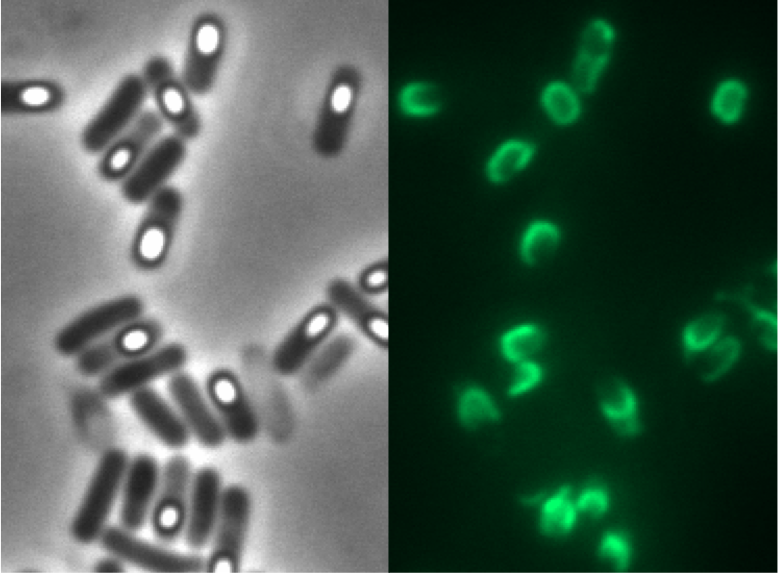
| - | <p align="justify">Finally, we started with the most important experiment for our GFP-'''Sporo'''beads, the fluorescence microscopy. We developed a sporulation protocol (for details see [https://static.igem.org/mediawiki/2012/e/e9/LMU-Munich_2012_Protocol_for_enhancement_of_mature_spore_numbers.pdf Protocol for enhancement of mature spore numbers]) that increases the rates of mature spores in our samples. The cells were fixed on agarose pads and investigated by phase contrast and fluorescence microscopy. While spores of the wild type only showed the known background fluorescence, all '''Sporo'''beads showed bright green fluorescence at the edge (on the surface) of the spores. '''Sporo'''beads from strain B 53 (containing the P<sub>''cotYZ''</sub>-''cotZ''-2aa-''gfp''-terminator construct) showed the highest fluorescence intensity (see Fig. 5 and all [https://2012.igem.org/Team:LMU-Munich/Data/gfp_spore data]). Hence, this strain was chosen for further experiments.</p>
| |
| - |
| |
| - |
| |
| - | {| style="color:black;" cellpadding="3" width="70%" cellspacing="0" border="0" align="center" style="text-align:center;"
| |
| - | | style="width: 70%;background-color: #EBFCE4;" |
| |
| - | {|
| |
| - | |[[File:WT-B53 fluorescence.png|300px|center]]
| |
| - | |-
| |
| - | | style="width: 70%;background-color: #EBFCE4;" |
| |
| - | {| style="color:black;" cellpadding="0" width="90%" cellspacing="0" border="0" align="center" style="text-align:center;"
| |
| - | |style="width: 70%;background-color: #EBFCE4;" |
| |
| - | <font color="#000000"; size="2"><p align="justify">Fig. 5: Fluorescence of wild type spores and B53-'''Sporo'''beads (containing the P<sub>''cotYZ''</sub>-''cotZ''-''gfp''-terminator construct)</p></font>
| |
| - | |}
| |
| - | |}
| |
| - | |}
| |
| - |
| |
| - |
| |
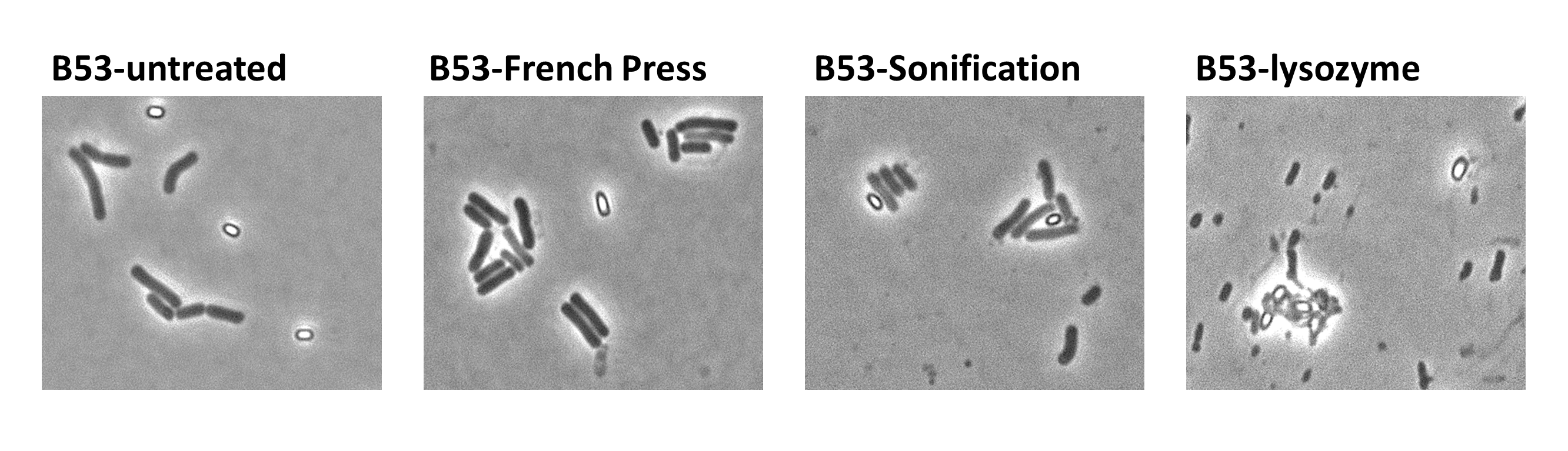
| - | <p align="justify">Since there were still some vegetative cells left after 24 hours of growth in Difco Sporulation Medium, we wanted to purify the '''Sporo'''beads. We tried three different methods for this approach: treatment with French Press, sonification and lysozyme. By means of the microscopy results we were able to conclude that lysozyme treatment was the only successful method (see [https://2012.igem.org/Team:LMU-Munich/Data/Sporepurification data]). Additionally, this treatment did not harm the crust fusion proteins as green fluorescence was still detectable afterwards (see [https://2012.igem.org/Team:LMU-Munich/Data/Sporepurification data] for details).</p>
| |
| - |
| |
| - | <p align="justify">Moreover, we wondered if clean deletions of the native spore crust genes would show any difference in fusion protein expression in our '''Sporo'''beads. Thus, we deleted the native ''cotZ'' and ''cgeA'' using the pMAD based gene deletion strategy described by [http://www.ncbi.nlm.nih.gov/pubmedterm=New%20Vector%20for%20Efficient%20Allelic%20Replacement%20in%20Naturally%20Nontransformable%2C%20Low-GC-Content%2C%20Gram-Positive%20Bacteria Arnaud ''et al''., 2004].</p>
| |
| - |
| |
| - |
| |
| - | <p align="justify">Because of the low but distinct fluorescence of wild type spores, we measured and compared the fluorescence intensity of 100 spores per construct (see [https://2012.igem.org/Team:LMU-Munich/Data/gfp_spore data]). We obtained significant differences between wild type spores and all of our '''Sporo'''beads (see [https://2012.igem.org/Team:LMU-Munich/Data/gfp_spore data]). The intensity bar charts in Fig. 6 show the fluorescence intensity, while the 3D graphs illustrate the distribution of fluorescence intensity across the spore surface. This correlates with the localization of our fusion proteins in the crust. For image analysis we measured the fluorescence intensity of an area of 750 pixel per spore by using ImageJ and evaluated the results with the statistical software R.
| |
| - | The following graph (Fig. 6) shows the results of microscopy and ImageJ analysis of the strongest construct integrated into wildtype W168 (B53) and the deletion strain B 49 (B70).</p>
| |
| - |
| |
| - |
| |
| - | {| style="color:black;" cellpadding="3" width="100%" cellspacing="0" border="0" align="center" style="text-align:left;"
| |
| - | | style="width: 70%;background-color: #EBFCE4;" |
| |
| - | {|
| |
| - | |[[File:Fluorescence of Sporobeads.png|610px|center]]
| |
| - | |}
| |
| - | |-
| |
| - | | style="width: 70%;background-color: #EBFCE4;" |
| |
| - | {| style="color:black;" cellpadding="0" width="100%" cellspacing="0" border="0" align="center" style="text-align:center;"
| |
| - | |style="width: 70%;background-color: #EBFCE4;" |
| |
| - | <font color="#000000"; size="2">Fig. 6: Result of fluorescence evaluation of the three strains W168, B53 and B70.</font>
| |
| - | |}
| |
| - | |}
| |
| - |
| |
| - |
| |
| - | <p align="justify">As shown in Fig. 6, the wild type spore has hardly any fluorescence, whereas both''' Sporo'''beads with the integrated construct pSB<sub>''Bs''</sub>1C-P<sub>''cotYZ''</sub>-''cotZ''<sub>-2aa</sub>-''gfp''-terminator give a distinct fluorescence signal around the edge of the spore. Furthermore, it demonstrates that strain B 70 has the highest fluorescence intensity.</p>
| |
| - |
| |
| - | <p align="justify">In summary we successfully developed functional sporobeads that are capable of displaying any protein of choice on the surface of modified ''B. subtilis'' endospores.</p>
| |
| - |
| |
| - |
| |
| | <div class="box"> | | <div class="box"> |
| | ====Project Navigation==== | | ====Project Navigation==== |
| Line 190: |
Line 81: |
| | |} | | |} |
| | </div> | | </div> |
| | + | |
| | + | |
| | | | |
| | <!-- Include the next line at the end of every page --> | | <!-- Include the next line at the end of every page --> |
| | {{:Team:LMU-Munich/Templates/Page Footer}} | | {{:Team:LMU-Munich/Templates/Page Footer}} |






 "
"