Team:LMU-Munich/Germination Stop
From 2012.igem.org
| Line 29: | Line 29: | ||
Further, we will investigate the possibility of using a redundant toxin system to immediately kill off any spores which somehow germinate. | Further, we will investigate the possibility of using a redundant toxin system to immediately kill off any spores which somehow germinate. | ||
| + | ====How does the GerminationSTOP aspect of the Beadzillus project actually work?==== | ||
| + | |||
| + | To understand how our germination knockouts actually prevent germination, it is crucial to understand how the ''Bacillus'' germination process occurs. | ||
| + | |||
| + | ''Bacillus'' cells form spores in a process called sporulation in response to starvation of nutrients (including carbon, nitrogen, or phosphorus) or in response to peptides secreted by other cells which signal too high of population densities to cells. | ||
| + | The “mother” cell forms the endospore within its own cell membrane. The spore contains its DNA in the spore core, which is protected by several layers of coats. The spore is very dry, and contains a substance called dipicolinic acid (DPA), which is replaced with water when the spore germinates. Until the spore is hydrated (and swells), it is resistant to a wide variety of environmental stressors, including UV radiation, toxic chemicals, freezing, high heat, dessication, and pH extremes. | ||
| + | |||
| + | [[File:sporulation.jpg]] | ||
| + | |||
| + | The spore has germinant receptors on its inner spore membrane, and is suspected to have semipermeable or porous outer layers that permit the passage of germinants to the receptors. When germinants such as amino acids and sugars reach germinant receptors, the spore begins a biochemical process of germination. It takes up water, shifts its pH, and swells. It breaks out of its coat and begins the outgrowth process. | ||
| + | |||
| + | [[File:outgrowth.jpg]] | ||
| + | |||
| + | We were concerned that because the beginning stage of germination is a strictly biochemical one, that maybe our spores would lose the ability to fully germinate, but would nonetheless become deformed by the initial steps of germination. This would be problematic because the spores should be vectors to carry proteins; deformed spores could be ineffective as delivery vectors. The investigative work of Plomp et al (2007) on ''Bacillus'' cells seems to support that the lytic enzyme knockouts we chose could help to maintain the spore shape. They state: | ||
| + | |||
| + | “A significant fraction (≈30%) of spores did not proceed to outgrowth in the timeframe of the observation and did not exhibit degradation of the rodlet layer. However, after drying, >90% of these spores showed a structural collapse, indicating prior replacement of the dipicolinic acid in the spore core with water, i.e. they did proceed through the germination stage, but not the outgrowth stage.” […] “Etch pits were the initiation sites for early germination-induced spore coat fissure formation.” […] “Disassembly of the higher-order rodlet structure initiates at micro-etch pits, and proceeds by the expansion of the pits to form fissures perpendicular to the rodlet direction.” […] “We suggest by analogy that rodlet structure degradation is caused by specific hydrolytic enzyme(s), located within the spore integument and activated during the early stages of germination.” | ||
| + | |||
| + | What we read from this is that spores can be cued to germinate, and the etch pits that lead to the destruction of the spore coat can be formed, but without the activity of lytic enzymes, further steps of germination do not occur. | ||
| + | |||
| + | [[File:germinationrates.jpg]] | ||
| + | |||
| + | Thus, by knocking out the genes coding for lytic enzymes, our goal is to prevent the disassembly of the spore coat. | ||
Revision as of 15:14, 6 September 2012

The LMU-Munich team is exuberantly happy about the great success at the World Championship Jamboree in Boston. Our project Beadzillus finished 4th and won the prize for the "Best Wiki" (with Slovenia) and "Best New Application Project".
[ more news ]

GerminationSTOP
The goal of this project is to remove the germination capability, while keeping the necessary structural functions of the spores intact.
There are two approaches to achive this:
- Knock out genes that are involved in germination.
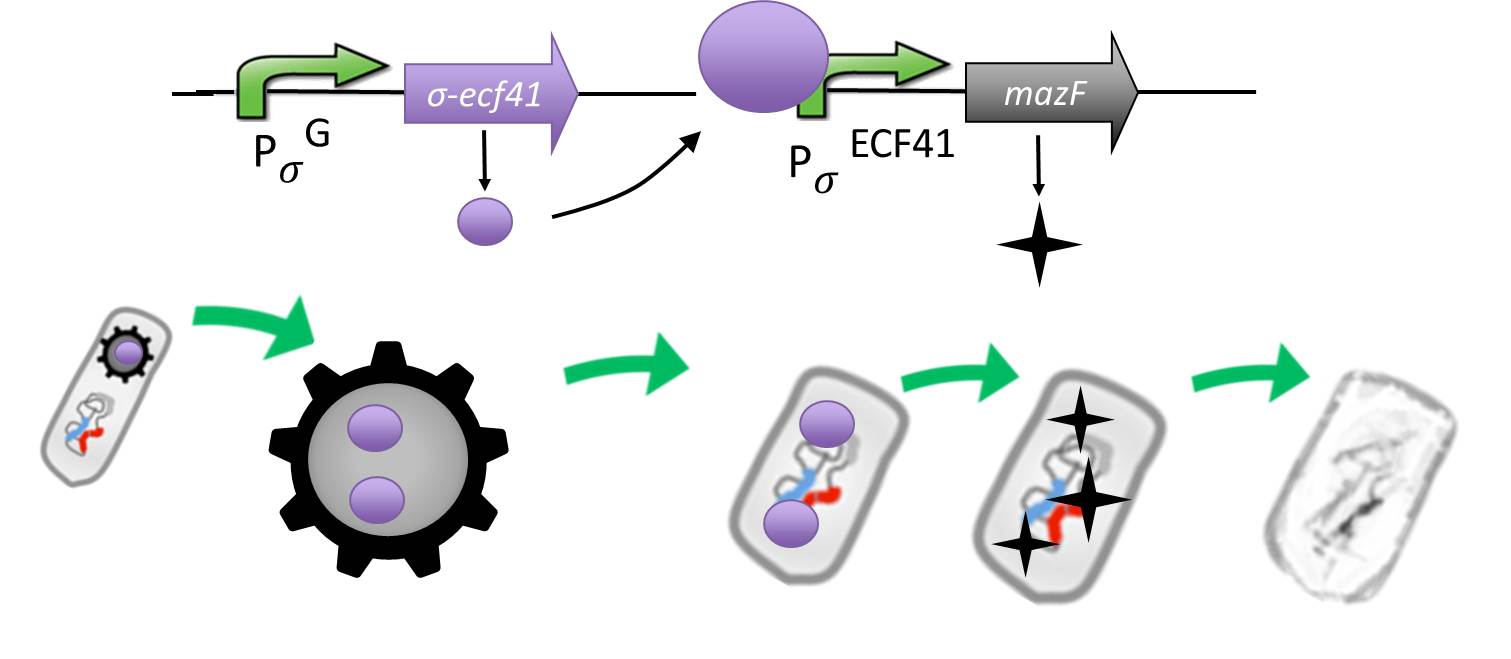
- Suicideswitch: Toxin production if germination knockout fails and spores manage to germinate.
Gene Knockouts
Based on the work of [http://www.ncbi.nlm.nih.gov/pubmed/19554258 J. Kim and W. Schumann (2009)] and [http://www.ncbi.nlm.nih.gov/pubmed/11466293 B. Setlow et al (2001)], we decided to knock out genes cwlJ, sleB, cwlB, gerD, and cwlD. The genes cwlJ and sleB code for lytic enzymes which are active in the process of germination. In the work of [http://www.ncbi.nlm.nih.gov/pubmed/11466293 B. Setlow et al (2001)], when cwlJ and sleB were knocked out together, germination frequency was reduced by 5 orders of magnitude. [http://www.ncbi.nlm.nih.gov/pubmed/19554258 J. Kim and W. Schumann (2009)] report a similar reduction in germination when gerD and cwlB are knocked out together, and a reduction in germination ability when the gene cwlD is knocked out. When all five of these genes are knocked out, we hope to yield a B. subtilis strain which produces spores completely incapable of germination.
Two methods are being employed to knock out germination: resistance cassette (RC) knockouts and clean deletions. Single RC knockouts were created, and have then been combined to create multiple knockouts.
This will be checked with germination assays.
Further, we will investigate the possibility of using a redundant toxin system to immediately kill off any spores which somehow germinate.
How does the GerminationSTOP aspect of the Beadzillus project actually work?
To understand how our germination knockouts actually prevent germination, it is crucial to understand how the Bacillus germination process occurs.
Bacillus cells form spores in a process called sporulation in response to starvation of nutrients (including carbon, nitrogen, or phosphorus) or in response to peptides secreted by other cells which signal too high of population densities to cells. The “mother” cell forms the endospore within its own cell membrane. The spore contains its DNA in the spore core, which is protected by several layers of coats. The spore is very dry, and contains a substance called dipicolinic acid (DPA), which is replaced with water when the spore germinates. Until the spore is hydrated (and swells), it is resistant to a wide variety of environmental stressors, including UV radiation, toxic chemicals, freezing, high heat, dessication, and pH extremes.
The spore has germinant receptors on its inner spore membrane, and is suspected to have semipermeable or porous outer layers that permit the passage of germinants to the receptors. When germinants such as amino acids and sugars reach germinant receptors, the spore begins a biochemical process of germination. It takes up water, shifts its pH, and swells. It breaks out of its coat and begins the outgrowth process.
We were concerned that because the beginning stage of germination is a strictly biochemical one, that maybe our spores would lose the ability to fully germinate, but would nonetheless become deformed by the initial steps of germination. This would be problematic because the spores should be vectors to carry proteins; deformed spores could be ineffective as delivery vectors. The investigative work of Plomp et al (2007) on Bacillus cells seems to support that the lytic enzyme knockouts we chose could help to maintain the spore shape. They state:
“A significant fraction (≈30%) of spores did not proceed to outgrowth in the timeframe of the observation and did not exhibit degradation of the rodlet layer. However, after drying, >90% of these spores showed a structural collapse, indicating prior replacement of the dipicolinic acid in the spore core with water, i.e. they did proceed through the germination stage, but not the outgrowth stage.” […] “Etch pits were the initiation sites for early germination-induced spore coat fissure formation.” […] “Disassembly of the higher-order rodlet structure initiates at micro-etch pits, and proceeds by the expansion of the pits to form fissures perpendicular to the rodlet direction.” […] “We suggest by analogy that rodlet structure degradation is caused by specific hydrolytic enzyme(s), located within the spore integument and activated during the early stages of germination.”
What we read from this is that spores can be cued to germinate, and the etch pits that lead to the destruction of the spore coat can be formed, but without the activity of lytic enzymes, further steps of germination do not occur.
Thus, by knocking out the genes coding for lytic enzymes, our goal is to prevent the disassembly of the spore coat.
Suicideswitch
Project Navigation
| +--+--+--+--+--+--+-- | +--+--+--+--+-- | +--+--+--+--+--+-- | +--+--+--+--+--+-- |

Bacillus BioBrickBOX |

SporeCoat FusionProteins |

Germination STOP |
 "
"