Team:LMU-Munich/Data/Knockout
From 2012.igem.org
| Line 7: | Line 7: | ||
===Knockouts of germination genes === | ===Knockouts of germination genes === | ||
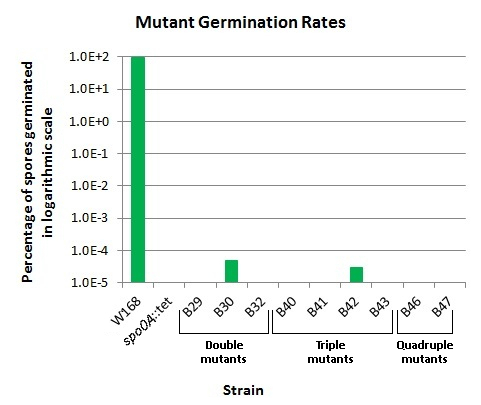
| - | <p align="justify">We incubated our mutant strains in Difco sporulation media (DSM) at 37°C | + | <p align="justify">We incubated our mutant strains in Difco sporulation media (DSM) shaking at 37°C for 18-24 hours to induce sporulation. We heated 1 mL of each of the DSM cultures at 80°C for one hour to kill vegetative cells, and used 1 mL at room temperature (allowing vegetative cells to survive). From here, we quantified the cells and spores per mL of each culture using a Neubauer cell counting chamber. Then on LB-agar, we plated different dilutions of the cultures, with heated and unheated cultures on separate plates. The purpose of plating both heated and unheated cultures was to demonstrate the viability of the unheated vegetative cells in each culture. This makes evident that the lack of colonies on heated plates is not due to the inability of the strain's vegetative cells to grow, but from the inability of spores to germinate into vegetative cells capable of growing. For all germination mutants, vegetative cells were able to grow and form colonies. Plates were incubated at 37°C for at least 24 hours. We then quantified the colonies on each plate, and used this to check for cell growth and calculate the spore germination rate for each mutant strain. For details on this germination assay, see [https://2012.igem.org/Team:LMU-Munich/Lab_Notebook/Protocols Protocols]. The germination assays were carried out several times, and yielded similar results each time. The results are shown in Fig. 1.</p> |
<br> | <br> | ||
| Line 33: | Line 33: | ||
|} | |} | ||
|} | |} | ||
| - | <p align="justify">The remaining germination ability in strain B30 in Fig. 1 seems inconsistent with the retained germination ability in strain B42 because B42 contains mutations to only one of the genes knocked out in B30. For example, we would have expected that B41 or B43, which contain both ''gerD''::cm and ''sleB''::mls should retain some germination ability instead. We could find no evidence of a labeling mix-up that could have caused the inconsistency. | + | <br> |
| + | <p align="justify">The remaining germination ability in strain B30 in Fig. 1 seems inconsistent with the retained germination ability in strain B42, because B42 contains mutations to only one of the genes knocked out in B30. For example, we would have expected that B41 or B43, which contain both ''gerD''::cm and ''sleB''::mls should retain some germination ability instead. We could find no evidence of a labeling mix-up that could have caused the inconsistency. But the germination of B42 spores occurred in only one of the many germination assays performed on this strain. Hence we cannot rule out an experimental artifact.</p> | ||
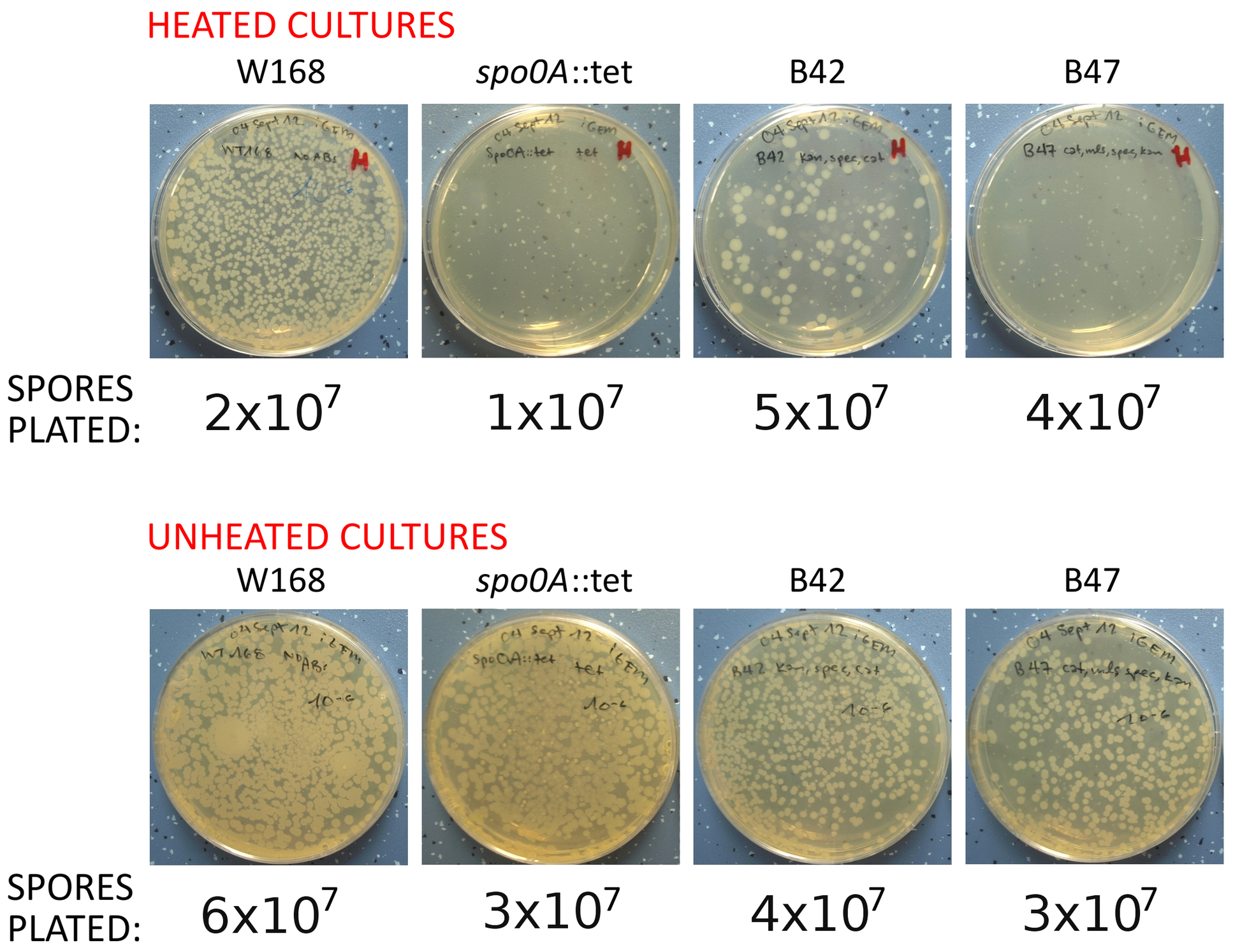
<p align="justify">The next figure showing the LB-agar plates from the germination assays (see below) demonstrates the inability of our mutant spores to germinate.</p> | <p align="justify">The next figure showing the LB-agar plates from the germination assays (see below) demonstrates the inability of our mutant spores to germinate.</p> | ||
| - | <p align="justify">The plates below | + | <p align="justify">The plates below illustrates the procedure of plating for the germination assay. Plates here include those containing heated and unheated cultures, and show both germinable spores (B42) and spores unable to germinate (B47). These plating experiments allowed us to quantify the number of ingerminable spores in each culture.</p> |
<br> | <br> | ||
| Line 56: | Line 57: | ||
<br> | <br> | ||
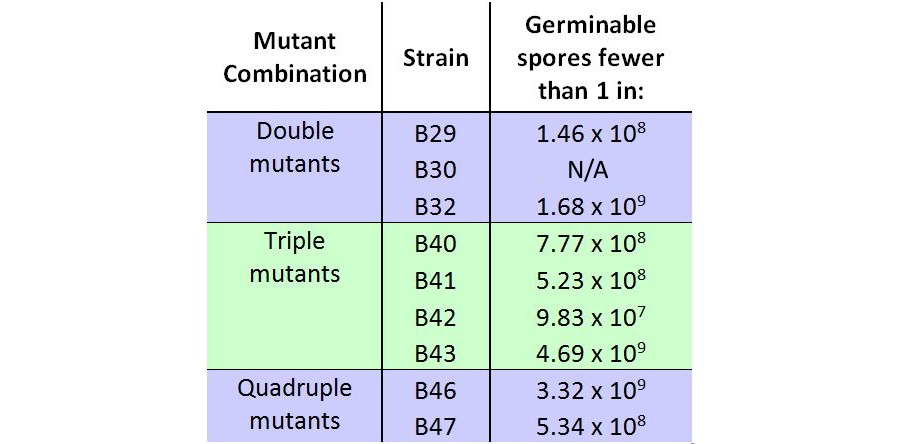
| - | <p align="justify">The table below shows the maximum number of spores from heated cultures plated which yielded zero colonies. In the case of B30, the germination assay was performed only once, and as visible in | + | <p align="justify">The table below shows the maximum number of spores from heated cultures plated which yielded zero colonies. In the case of B30, the germination assay was performed only once, and as visible in Fig. 2, contained viable, germinable spores. The numbers shown here are the highest number of spores plated, excluding B30. This means that we do not have data for higher concentrations of spores which would suggest any spore germinability. The mutant strain B42 contained germinable spores when 4x10<sup>7</sup> were plated, but contrarily, zero germinable spores were found when 9.83x10<sup>7</sup> spores were plated in another round of the germination assay.</p> |
<br> | <br> | ||
Revision as of 23:11, 26 September 2012
The LMU-Munich team is exuberantly happy about the great success at the World Championship Jamboree in Boston. Our project Beadzillus finished 4th and won the prize for the "Best Wiki" (with Slovenia) and "Best New Application Project".
[ more news ]

Knockouts of germination genes
We incubated our mutant strains in Difco sporulation media (DSM) shaking at 37°C for 18-24 hours to induce sporulation. We heated 1 mL of each of the DSM cultures at 80°C for one hour to kill vegetative cells, and used 1 mL at room temperature (allowing vegetative cells to survive). From here, we quantified the cells and spores per mL of each culture using a Neubauer cell counting chamber. Then on LB-agar, we plated different dilutions of the cultures, with heated and unheated cultures on separate plates. The purpose of plating both heated and unheated cultures was to demonstrate the viability of the unheated vegetative cells in each culture. This makes evident that the lack of colonies on heated plates is not due to the inability of the strain's vegetative cells to grow, but from the inability of spores to germinate into vegetative cells capable of growing. For all germination mutants, vegetative cells were able to grow and form colonies. Plates were incubated at 37°C for at least 24 hours. We then quantified the colonies on each plate, and used this to check for cell growth and calculate the spore germination rate for each mutant strain. For details on this germination assay, see Protocols. The germination assays were carried out several times, and yielded similar results each time. The results are shown in Fig. 1.
|
The remaining germination ability in strain B30 in Fig. 1 seems inconsistent with the retained germination ability in strain B42, because B42 contains mutations to only one of the genes knocked out in B30. For example, we would have expected that B41 or B43, which contain both gerD::cm and sleB::mls should retain some germination ability instead. We could find no evidence of a labeling mix-up that could have caused the inconsistency. But the germination of B42 spores occurred in only one of the many germination assays performed on this strain. Hence we cannot rule out an experimental artifact.
The next figure showing the LB-agar plates from the germination assays (see below) demonstrates the inability of our mutant spores to germinate.
The plates below illustrates the procedure of plating for the germination assay. Plates here include those containing heated and unheated cultures, and show both germinable spores (B42) and spores unable to germinate (B47). These plating experiments allowed us to quantify the number of ingerminable spores in each culture.
|
The table below shows the maximum number of spores from heated cultures plated which yielded zero colonies. In the case of B30, the germination assay was performed only once, and as visible in Fig. 2, contained viable, germinable spores. The numbers shown here are the highest number of spores plated, excluding B30. This means that we do not have data for higher concentrations of spores which would suggest any spore germinability. The mutant strain B42 contained germinable spores when 4x107 were plated, but contrarily, zero germinable spores were found when 9.83x107 spores were plated in another round of the germination assay.
|
 "
"