Team:HokkaidoU Japan/Notebook/aggregation Week 8
From 2012.igem.org
(mini-prep image) |
|||
| Line 237: | Line 237: | ||
#Added 1 ml LBK in one culture as negative control. | #Added 1 ml LBK in one culture as negative control. | ||
#Added 900 ul LBK and 100 ul 20% L-arabinose. | #Added 900 ul LBK and 100 ul 20% L-arabinose. | ||
| - | #Incubated at 37C | + | #Incubated at 37C 130 rpm for 2 hours and 30 minutes. |
| - | #Placed tubes on the table at | + | #Placed tubes on the table at 30 minutes. |
</p> | </p> | ||
==Mini-prep== | ==Mini-prep== | ||
<p> | <p> | ||
| - | Mni-prep of pT7-RBS on pSB1C3 of colony No.4 and 5 selected by the result of colony PCR yesterday. We used mini-prep kit of Nippon genetics: FastGene Plasmid mini kit and finally we eluted the DNA with | + | Mni-prep of pT7-RBS on pSB1C3 of colony No. 4 and 5 selected by the result of colony PCR yesterday. We used mini-prep kit of Nippon genetics: FastGene Plasmid mini kit and finally we eluted the DNA with 20 ul of elution buffer. |
| - | [[image:HokkaidoU2012 120821 pT7-RBS on pSB1C3 ColonyNo.4,5 mini-prep.jpg|thumb|mini-prep result]] | + | [[image:HokkaidoU2012 120821 pT7-RBS on pSB1C3 ColonyNo. 4,5 mini-prep.jpg|thumb|mini-prep result]] |
| - | The concentration of | + | The concentration of 20 ul of mini-prep products were low to digestion or do something so we retry liquid culture of other number of colony solution: No. 1 and No. 2. |
</p> | </p> | ||
| Line 255: | Line 255: | ||
==liquid culture== | ==liquid culture== | ||
<p> | <p> | ||
| - | Liquid culture for Ag43-dT on pSB1AK3 (and pT7-RBS on pSB1C3 colony No.1 and 2 which were selected from the results of colony PCR). | + | Liquid culture for Ag43-dT on pSB1AK3 (and pT7-RBS on pSB1C3 colony No. 1 and 2 which were selected from the results of colony PCR). |
#Added 2 ml of LBA (LBC) into culture tubes. | #Added 2 ml of LBA (LBC) into culture tubes. | ||
| - | #Resuspended 2 colonies (Resuspended pre-cultivated | + | #Resuspended 2 colonies (Resuspended pre-cultivated 200 ul of LB and colony solution). |
#Incubated the tubes at 37C for 18 hours (16 hours). | #Incubated the tubes at 37C for 18 hours (16 hours). | ||
</p> | </p> | ||
| Line 269: | Line 269: | ||
==Mini-prep== | ==Mini-prep== | ||
<p> | <p> | ||
| - | Mni-prep of pT7-RBS on pSB1C3 of colony No.1 and 2 selected by the result of colony PCR in 20th. We used mini-prep kit of Nippon genetics: FastGene Plasmid mini kit and finally we eluted the DNA with | + | Mni-prep of pT7-RBS on pSB1C3 of colony No.1 and 2 selected by the result of colony PCR in 20th. We used mini-prep kit of Nippon genetics: FastGene Plasmid mini kit and finally we eluted the DNA with 20 ul of elution buffer. |
| - | + | ||
[[image:HokkaidoU2012 120822 pT7-RBS on pSB1C3 mini-prep No.jpg|thumb|mini-prep result]] | [[image:HokkaidoU2012 120822 pT7-RBS on pSB1C3 mini-prep No.jpg|thumb|mini-prep result]] | ||
| - | + | ||
| + | |||
| + | |||
| + | One of Ag43-dT on pSB1AK3 culture did not get myddy. And another one is only a little muddy. | ||
| + | We tried mini-prep to the latter, we god the 20 ul of DNA solution. | ||
| + | And then, we did electrophoresis the mini-prep products and (pBAD-RBS and pBAD) gel extract products. | ||
| + | |||
| + | [[image:|thumb|electrophoresis result]] | ||
</p> | </p> | ||
| - | </ | + | |
| + | ==PCR== | ||
| + | <p> | ||
| + | PCR of pT7-RBS on pSB1C3.<br /> | ||
| + | We used 4 kinds of primer set. | ||
| + | |||
| + | 1 : EX-F , PS-R primer | ||
| + | 2 : EX-F , 200b down primer | ||
| + | 3 : 100b up , PS-R primer | ||
| + | 4 : 100b up , 200b down primer | ||
| + | |||
| + | The density of primer solutions is 10 uM. | ||
| + | |||
| + | {|class="hokkaidou-table-pcr-reagent" | ||
| + | |- | ||
| + | |DNA solution | ||
| + | |1 ul | ||
| + | |- | ||
| + | |KOD-Plus-NEO(Taq polymerase) | ||
| + | |1 ul | ||
| + | |- | ||
| + | |dNTP | ||
| + | |5 ul | ||
| + | |- | ||
| + | |MgSO4 | ||
| + | |3 ul | ||
| + | |- | ||
| + | |KOD-Plus-NEO Buffer | ||
| + | |5 ul | ||
| + | |- | ||
| + | |Forward Primer | ||
| + | |1 ul | ||
| + | |- | ||
| + | |Reverse Primer | ||
| + | |1 ul | ||
| + | |- | ||
| + | |DW | ||
| + | |33 ul | ||
| + | |- | ||
| + | |Total | ||
| + | |50 ul | ||
| + | |} | ||
| + | |||
| + | |||
| + | {|class="hokkaidou-table-pcr-time" | ||
| + | |- | ||
| + | |Number | ||
| + | |Degree | ||
| + | |Second | ||
| + | |- | ||
| + | |1 | ||
| + | |94 | ||
| + | |120 | ||
| + | |- | ||
| + | |2 | ||
| + | |98 | ||
| + | |10 | ||
| + | |- | ||
| + | |3 | ||
| + | |58 | ||
| + | |30 | ||
| + | |- | ||
| + | |4 | ||
| + | |68 | ||
| + | |30 | ||
| + | |- | ||
| + | |5 | ||
| + | |4 | ||
| + | |HOLD | ||
| + | |} | ||
| + | Cycle:2~4 x 45 | ||
| + | |||
| + | |||
| + | |||
| + | [[image:|thumb|PCR result]] | ||
| + | |||
| + | </div><div> | ||
| + | |||
<!-- DO NOT EDIT UNDER THIS LINE @iTakeshi --> | <!-- DO NOT EDIT UNDER THIS LINE @iTakeshi --> | ||
</div> | </div> | ||
<br style="line-height: 0; clear: both;" /> | <br style="line-height: 0; clear: both;" /> | ||
{{Team:HokkaidoU_Japan/footer}} | {{Team:HokkaidoU_Japan/footer}} | ||
Revision as of 08:37, 22 August 2012
Contents |
August 20th
Single colony isolation
Single colony isolation of pBAD-RBS-Ag43-dT on pSB1AK3.
- Picked up one colony.
- Cultivation on LBK(dt,RBS,T7) and LBK(pLacI-RBS-Ag43) in hours.
Colony PCR
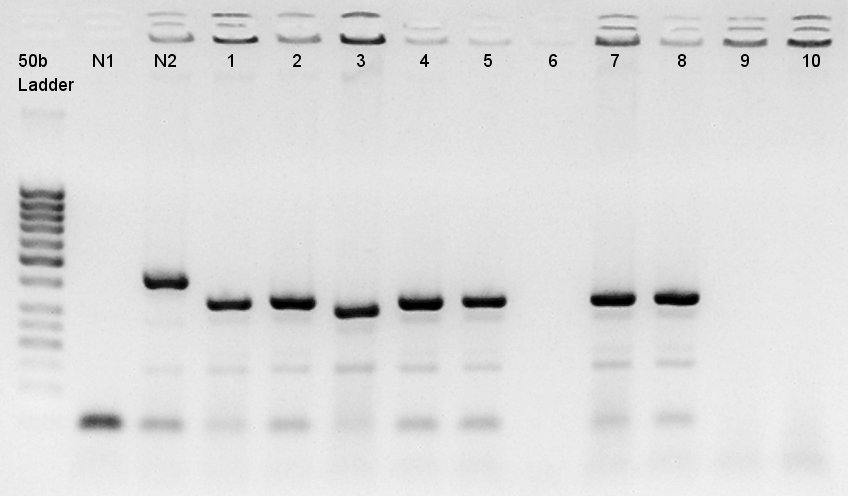
Colony PCR to confirm that whether the pT7 and RBS was successfully ligated with pSB1C3 or not.
| DNA solution | 4 ul |
| Kapa-Taq(Taq polymerase) | 5 ul |
| Forward Primer(100bp up primer) | 0.5 ul |
| Reverse Primer(200bp down primer) | 0.5 ul |
| Total | 10 ul |
| Number | Degree | Second |
| 1 | 95 | 120 |
| 2 | 95 | 30 |
| 3 | 53.2 | 30 |
| 4 | 72 | 60 |
| 5 | 72 | 60 |
| 6 | 4 | HOLD |
Cycle:2~4 x 35
We used N1 (DW only) and N2 (pBAD(containing araC)-RBS on pSB1A3) as controls. Desired product is about 300~400bp.
The results showed that ligated DNA has 300 ~ 400 bp and the desired products would have 331bp if it were amplified by 100bp up primer and 200bp down primer. Thus we confirmed that pT7-RBS on pSB1C3 was successfully ligated without no.6,9,10 colonies, but these 3 solution were evapolated because of our mistake. We selected No.4 and 5 colony for liquid culture.
PCR
PCR of BBa_I13453 (pBAD only part, it is not contain araC,).
| DNA solution | 1 ul |
| KOD-Plus-NEO(Taq polymerase) | 1 ul |
| dNTP | 5 ul |
| MgSO4 | 3 ul |
| KOD-Plus-NEO Buffer | 5 ul |
| Forward Primer(Ag43-f4 primer: 10 uM) | 1 ul |
| Reverse Primer(PS-R primer: 10 uM) | 1 ul |
| DW | 33 ul |
| Total | 50 ul |
| Number | Degree | Second |
| 1 | 94 | 120 |
| 2 | 98 | 10 |
| 3 | 58 | 30 |
| 4 | 68 | 30 |
| 5 | 4 | HOLD |
Cycle:2~4 x 45
[[image:|thumb|PCR result]]
liquid culture
Liquid culture for 3 colonies of pBAD-RBS-Ag43-dT on pSB1AK3 (and pT7-RBS on pSB1C3 colony No.4 and 5 which were selected from the results of colony PCR).
- Added 2 ml of LBK (LBC) into culture tubes.
- Resuspended 1 colonies (Resuspended pre-cultivated 200ul of LB and colony solution).
- Incubated the tubes at 37C for 16 hours (19 hours).
August 21th
PCR
PCR of pBAD(containing araC)-RBS. And, we checked the plasmid which we did mini-prep at August 18th is pBAD-RBS on pSB1A3 or not.
| DNA solution | 1 ul |
| KOD-Plus-NEO(Taq polymerase) | 1 ul |
| dNTP | 5 ul |
| MgSO4 | 3 ul |
| KOD-Plus-NEO Buffer | 5 ul |
| Forward Primer(Ag43-f4 primer: 10 uM) | 1 ul |
| Reverse Primer(PS-R primer: 10 uM) | 1 ul |
| DW | 33 ul |
| Total | 50 ul |
| Number | Degree | Second |
| 1 | 94 | 120 |
| 2 | 98 | 10 |
| 3 | 58 | 30 |
| 4 | 68 | 30 |
| 5 | 4 | HOLD |
Cycle:2~4 x 45
[[image:|thumb|PCR result]]
Aggregation check
we cultured the E. coli, which transformed pBAD-RBS-Ag43-dT on pSB1AK3 in LBK. We checked the construct by induction of L-arabinose after 16 hours incubate.
- 2 ml of liquid culture divided two culture. (made two 1 ml culture)
- Added 1 ml LBK in one culture as negative control.
- Added 900 ul LBK and 100 ul 20% L-arabinose.
- Incubated at 37C 130 rpm for 2 hours and 30 minutes.
- Placed tubes on the table at 30 minutes.
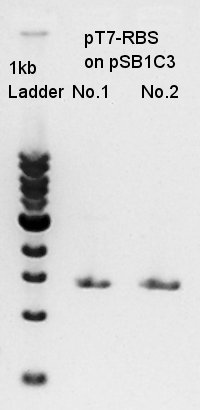
Mini-prep
Mni-prep of pT7-RBS on pSB1C3 of colony No. 4 and 5 selected by the result of colony PCR yesterday. We used mini-prep kit of Nippon genetics: FastGene Plasmid mini kit and finally we eluted the DNA with 20 ul of elution buffer.
The concentration of 20 ul of mini-prep products were low to digestion or do something so we retry liquid culture of other number of colony solution: No. 1 and No. 2.
liquid culture
Liquid culture for Ag43-dT on pSB1AK3 (and pT7-RBS on pSB1C3 colony No. 1 and 2 which were selected from the results of colony PCR).
- Added 2 ml of LBA (LBC) into culture tubes.
- Resuspended 2 colonies (Resuspended pre-cultivated 200 ul of LB and colony solution).
- Incubated the tubes at 37C for 18 hours (16 hours).
August 22th
Mini-prep
Mni-prep of pT7-RBS on pSB1C3 of colony No.1 and 2 selected by the result of colony PCR in 20th. We used mini-prep kit of Nippon genetics: FastGene Plasmid mini kit and finally we eluted the DNA with 20 ul of elution buffer.
One of Ag43-dT on pSB1AK3 culture did not get myddy. And another one is only a little muddy. We tried mini-prep to the latter, we god the 20 ul of DNA solution. And then, we did electrophoresis the mini-prep products and (pBAD-RBS and pBAD) gel extract products.
[[image:|thumb|electrophoresis result]]
PCR
PCR of pT7-RBS on pSB1C3.
We used 4 kinds of primer set.
1 : EX-F , PS-R primer
2 : EX-F , 200b down primer
3 : 100b up , PS-R primer
4 : 100b up , 200b down primer
The density of primer solutions is 10 uM.
| DNA solution | 1 ul |
| KOD-Plus-NEO(Taq polymerase) | 1 ul |
| dNTP | 5 ul |
| MgSO4 | 3 ul |
| KOD-Plus-NEO Buffer | 5 ul |
| Forward Primer | 1 ul |
| Reverse Primer | 1 ul |
| DW | 33 ul |
| Total | 50 ul |
| Number | Degree | Second |
| 1 | 94 | 120 |
| 2 | 98 | 10 |
| 3 | 58 | 30 |
| 4 | 68 | 30 |
| 5 | 4 | HOLD |
Cycle:2~4 x 45
[[image:|thumb|PCR result]]
</div>
</div>
</div>
</div>
 "
"