Team:HokkaidoU Japan/Notebook/aggregation Week 7
From 2012.igem.org
| Line 4: | Line 4: | ||
<!-- DO NOT EDIT OVER THIS LINE @iTakeshi --> | <!-- DO NOT EDIT OVER THIS LINE @iTakeshi --> | ||
<div class="hokkaidou-notebook-daily"> | <div class="hokkaidou-notebook-daily"> | ||
| - | ==August 16th== | + | ===August 16th=== |
| - | <div> | + | <div class="hokkaidou-section"> |
| - | ==ligation== | + | ====ligation==== |
| - | + | ||
We ligated pBad-RBS on pSB1A3 (digested Spe1) as vector and Ag43-dT (digested Spe1 and Xba1 and HindIII) as insert. | We ligated pBad-RBS on pSB1A3 (digested Spe1) as vector and Ag43-dT (digested Spe1 and Xba1 and HindIII) as insert. | ||
| - | |||
| - | |||
{|class="hokkaidou-table-ligation" | {|class="hokkaidou-table-ligation" | ||
|- | |- | ||
| Line 31: | Line 28: | ||
Ligation reaction time was written below. | Ligation reaction time was written below. | ||
| - | |||
{|class="hokkaidou-table-ligation" | {|class="hokkaidou-table-ligation" | ||
|- | |- | ||
| Line 46: | Line 42: | ||
|Hold | |Hold | ||
|} | |} | ||
| - | |||
| - | ==Transformation== | + | ====Transformation==== |
| - | + | ||
Transformation of plasmid DNA ligation product (pT7-RBS-Ag43-dT on pSB1K3) into DH5α. | Transformation of plasmid DNA ligation product (pT7-RBS-Ag43-dT on pSB1K3) into DH5α. | ||
#Added 2 ul of plasmid DNA to 50 ul of thawed competent cells on ice. | #Added 2 ul of plasmid DNA to 50 ul of thawed competent cells on ice. | ||
| Line 58: | Line 52: | ||
#Added 450 ul of LB to 50 ul of the transformation and plated 300 ul of it onto second dish and spread. | #Added 450 ul of LB to 50 ul of the transformation and plated 300 ul of it onto second dish and spread. | ||
#Incubated the plates at 37C for hours. | #Incubated the plates at 37C for hours. | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | Ag43-dT | + | ====Digestion==== |
| - | SpeI and XbaI | + | Ag43-dT on pSB1AK3 digestion with SpeI and XbaI.<br /> |
| + | Ag43-dT, SpeI and XbaI | ||
{|class="hokkaidou-table-digestion" | {|class="hokkaidou-table-digestion" | ||
|- | |- | ||
| Line 87: | Line 77: | ||
|} | |} | ||
| - | + | ||
[[image:HokaidoU2012_120813_pbad-rbs-ori-x10.jpg|thumb|pBad-RBS on pSB1A3 electrophoresis result]] | [[image:HokaidoU2012_120813_pbad-rbs-ori-x10.jpg|thumb|pBad-RBS on pSB1A3 electrophoresis result]] | ||
[[image:HokkaidoU2012_120813_ag43-dt-s-x.jpg|thumb|digestion result]] | [[image:HokkaidoU2012_120813_ag43-dt-s-x.jpg|thumb|digestion result]] | ||
| - | ==Gel extraction== | + | ====Gel extraction==== |
| - | + | ||
Gel extraction for digestion product. We used FastGene Gel&PCR extraction kit(NipponGenetics)and got 50 ul of DNA solution. | Gel extraction for digestion product. We used FastGene Gel&PCR extraction kit(NipponGenetics)and got 50 ul of DNA solution. | ||
| - | |||
</div></div> | </div></div> | ||
| - | |||
<div class="hokkaidou-notebook-daily"> | <div class="hokkaidou-notebook-daily"> | ||
| - | ==August 17th== | + | ===August 17th=== |
| - | <div> | + | <div class="hokkaidou-section"> |
| - | ==Colony PCR== | + | ====Colony PCR==== |
| - | + | ||
Colony PCR to confirm that whether the Ag43-dT was successfully ligated with pBAD-RBS on pSB1A3 or not. | Colony PCR to confirm that whether the Ag43-dT was successfully ligated with pBAD-RBS on pSB1A3 or not. | ||
| - | |||
| - | |||
{|class="hokkaidou-table-pcr-reagent" | {|class="hokkaidou-table-pcr-reagent" | ||
|- | |- | ||
| Line 166: | Line 150: | ||
We cultured these DNA in E. coli solution, after added 200 ul LB, at 37C for 3 hrs. | We cultured these DNA in E. coli solution, after added 200 ul LB, at 37C for 3 hrs. | ||
Next step, we resuspended these 5 colonies and cultured (add 1800 ul LB and 2 ul Amp) for hours at 37C. | Next step, we resuspended these 5 colonies and cultured (add 1800 ul LB and 2 ul Amp) for hours at 37C. | ||
| - | |||
| - | ==Transformation== | + | ====Transformation==== |
| - | + | ||
Transformation of BBa_I13453 (pBAD on pSB1A3) into DH5α. | Transformation of BBa_I13453 (pBAD on pSB1A3) into DH5α. | ||
#Added 1 ul of plasmid DNA to 50 ul of thawed competent cells on ice. | #Added 1 ul of plasmid DNA to 50 ul of thawed competent cells on ice. | ||
| Line 178: | Line 160: | ||
#Added 450 ul of LB to 50 ul of the transformation and plated 300 ul of it onto second dish and spread. | #Added 450 ul of LB to 50 ul of the transformation and plated 300 ul of it onto second dish and spread. | ||
#Incubated the plates at 37C for 19 hours. | #Incubated the plates at 37C for 19 hours. | ||
| - | |||
| - | ==Digestion== | + | ====Digestion==== |
| - | + | ||
From sequencing results, we found that we failed to make pT7-RBS on pSB1K3.<br /> | From sequencing results, we found that we failed to make pT7-RBS on pSB1K3.<br /> | ||
So, we tried to make it again. <br /> | So, we tried to make it again. <br /> | ||
| Line 254: | Line 234: | ||
|20 ul | |20 ul | ||
|} | |} | ||
| - | |||
| - | |||
</div></div> | </div></div> | ||
| - | |||
<div class="hokkaidou-notebook-daily"> | <div class="hokkaidou-notebook-daily"> | ||
| - | + | ===August 18th=== | |
| - | ==August 18th== | + | <div class="hokkaidou-section"> |
| - | <div> | + | ====Ethanol precipitation==== |
| - | ==Ethanol precipitation== | + | |
| - | + | ||
Ethanol precipitation for three digestion products. | Ethanol precipitation for three digestion products. | ||
#Added 2 ul of NaoAc, 1.5 ul of glycogen and 50 ul of 100% ethanol. | #Added 2 ul of NaoAc, 1.5 ul of glycogen and 50 ul of 100% ethanol. | ||
| Line 271: | Line 246: | ||
#Centrifuged at 15000 rpm, 15 min at 4C. | #Centrifuged at 15000 rpm, 15 min at 4C. | ||
#Removed supernatant and dried out at room temperature, after that added 10 ul of DW. | #Removed supernatant and dried out at room temperature, after that added 10 ul of DW. | ||
| - | |||
| - | ==Gel extraction== | + | ====Gel extraction==== |
| - | + | ||
Before digestion of pBAD-RBS on pSB1A3 and Ag43-dT on pSB1AK3, it is necessary to refine these two DNA. We used FastGene Gel&PCR extraction kit(NipponGenetics)and got 20 ul of DNA solution from 10 ul of plasmid extraction products. | Before digestion of pBAD-RBS on pSB1A3 and Ag43-dT on pSB1AK3, it is necessary to refine these two DNA. We used FastGene Gel&PCR extraction kit(NipponGenetics)and got 20 ul of DNA solution from 10 ul of plasmid extraction products. | ||
| - | + | ||
| - | ==Digestion== | + | ====Digestion==== |
| - | + | ||
Digested pBAD-RBS on pSB1A3 as insert and Ag43-dT on pSB1AK3 as vector with EcoRI and SpeI to make a constract, pBAD-RBS-Ag43-dT on pSB1AK3.<br /> | Digested pBAD-RBS on pSB1A3 as insert and Ag43-dT on pSB1AK3 as vector with EcoRI and SpeI to make a constract, pBAD-RBS-Ag43-dT on pSB1AK3.<br /> | ||
<br /> | <br /> | ||
| - | |||
pBAD-RBS<br /> | pBAD-RBS<br /> | ||
EcoRI/SpeI | EcoRI/SpeI | ||
| Line 330: | Line 301: | ||
[[image:HokkaidoU2012_120818_pbad-rbs--ag43-dt-dig.jpg|thumb|Digestion result]] | [[image:HokkaidoU2012_120818_pbad-rbs--ag43-dt-dig.jpg|thumb|Digestion result]] | ||
| - | |||
| - | ==Gel extraction== | + | ====Gel extraction==== |
| - | + | ||
Gel extraction for digestion product. We used FastGene Gel&PCR extraction kit(NipponGenetics)and got 50 ul of DNA solution. | Gel extraction for digestion product. We used FastGene Gel&PCR extraction kit(NipponGenetics)and got 50 ul of DNA solution. | ||
| - | |||
| - | ==Ethanol precipitation== | + | ====Ethanol precipitation==== |
| - | + | ||
Ethanol precipitation for digestion products. | Ethanol precipitation for digestion products. | ||
#Added 5 ul of NaoAc, 1.5 ul of glycogen and 125 ul of 100% ethanol. | #Added 5 ul of NaoAc, 1.5 ul of glycogen and 125 ul of 100% ethanol. | ||
| Line 345: | Line 312: | ||
#Centrifuged at 15000 rpm, 5 min at 4C. | #Centrifuged at 15000 rpm, 5 min at 4C. | ||
#Removed supernatant and dried out at room temperature, after that added 10 ul of DW. | #Removed supernatant and dried out at room temperature, after that added 10 ul of DW. | ||
| - | |||
| - | ==Plasmid extraction== | + | ====Plasmid extraction==== |
| - | + | ||
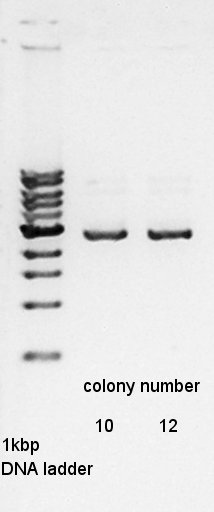
Plasmid extraction for colony number of No. 10 and 12 of pBAD-RBS-Ag43-dT. We used FastGene Plasmid Mini Kit(Nippon Genetics) and got 50 ul of DNA solutions. | Plasmid extraction for colony number of No. 10 and 12 of pBAD-RBS-Ag43-dT. We used FastGene Plasmid Mini Kit(Nippon Genetics) and got 50 ul of DNA solutions. | ||
[[image:HokkaidoU2012_120818_pbad-rbs-ag43-dt.jpg|thumb|plasmid extraction result]] | [[image:HokkaidoU2012_120818_pbad-rbs-ag43-dt.jpg|thumb|plasmid extraction result]] | ||
| - | |||
| - | ==Ligation== | + | ====Ligation==== |
| - | + | ||
Ligation for pT7 and RBS as inserts and pSB1C3 as vector. | Ligation for pT7 and RBS as inserts and pSB1C3 as vector. | ||
| - | |||
{|class="hokkaidou-table-ligation" | {|class="hokkaidou-table-ligation" | ||
|- | |- | ||
| Line 425: | Line 387: | ||
|Hold | |Hold | ||
|} | |} | ||
| - | |||
| - | |||
</div></div> | </div></div> | ||
| - | |||
<div class="hokkaidou-notebook-daily"> | <div class="hokkaidou-notebook-daily"> | ||
| - | ==August 19th== | + | ===August 19th=== |
| - | <div> | + | <div class="hokkaidou-section"> |
| - | ==Transformation== | + | ====Transformation==== |
| - | + | ||
Transformation of ligation product (two construct ligated at August 18) into DH5α.<br /> | Transformation of ligation product (two construct ligated at August 18) into DH5α.<br /> | ||
| - | |||
| - | |||
pT7-RBS on pSB1C3 | pT7-RBS on pSB1C3 | ||
#Added 2 ul of plasmid DNA to 50 ul of thawed competent cells on ice. | #Added 2 ul of plasmid DNA to 50 ul of thawed competent cells on ice. | ||
| Line 457: | Line 413: | ||
#Added 450 ul of LB to 50 ul of the transformation and plated 300 ul of it onto second dish and spread. | #Added 450 ul of LB to 50 ul of the transformation and plated 300 ul of it onto second dish and spread. | ||
#Incubated the plates at 37C for 16 hours and 30 minutes. | #Incubated the plates at 37C for 16 hours and 30 minutes. | ||
| - | |||
| - | ==PCR== | + | ====PCR==== |
| - | + | ||
PCR for pT7-RBS on pSB1K3. | PCR for pT7-RBS on pSB1K3. | ||
By sequencing reaction, we found the No. 10 plasmid is not pT7-RBS on pSB1K3. | By sequencing reaction, we found the No. 10 plasmid is not pT7-RBS on pSB1K3. | ||
| Line 524: | Line 478: | ||
No. 4 plasmid has independent bands. | No. 4 plasmid has independent bands. | ||
However, if it is pT7-RBS on pSB1K3, it has about 60~70bp band. | However, if it is pT7-RBS on pSB1K3, it has about 60~70bp band. | ||
| - | + | </div><div class="hokkaidou-section"> | |
| - | + | ||
| - | + | ||
| - | </div><div> | + | |
<!-- DO NOT EDIT UNDER THIS LINE @iTakeshi --> | <!-- DO NOT EDIT UNDER THIS LINE @iTakeshi --> | ||
Revision as of 02:29, 27 September 2012
Contents |
August 16th
ligation
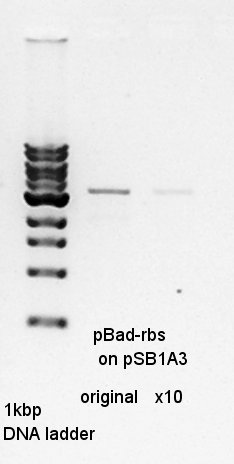
We ligated pBad-RBS on pSB1A3 (digested Spe1) as vector and Ag43-dT (digested Spe1 and Xba1 and HindIII) as insert.
| pT7-RBS (5 ng/ul) | 1 ul |
| Ag43-dT (25 ng/ul) | 2 ul |
| Ligation Mighty Mix(TAKARA) | 5 ul |
| DW | 2 ul |
| Total | 10 ul |
Ligation reaction time was written below.
| Degree | Minute |
| 16 | 30 |
| 65 | 10 |
| 4 | Hold |
Transformation
Transformation of plasmid DNA ligation product (pT7-RBS-Ag43-dT on pSB1K3) into DH5α.
- Added 2 ul of plasmid DNA to 50 ul of thawed competent cells on ice.
- Incubated on ice for 30min.
- Added 350 ul of LB.
- Prepared and Labeled two petri dishes with LBA.
- Plate 300 ul of the transformation onto first dish and spread.
- Added 450 ul of LB to 50 ul of the transformation and plated 300 ul of it onto second dish and spread.
- Incubated the plates at 37C for hours.
Digestion
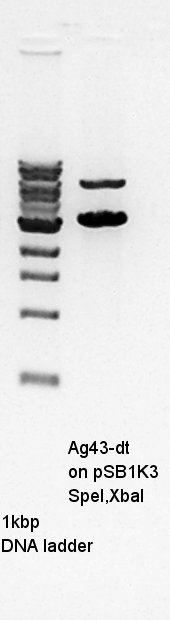
Ag43-dT on pSB1AK3 digestion with SpeI and XbaI.
Ag43-dT, SpeI and XbaI
| DNA solution (100 ng/ul) | 12 ul |
| SpeI | 1 ul |
| XbaI | 1 ul |
| 10xH buffer | 2 ul |
| DW | 4 ul |
| Total | 20 ul |
Gel extraction
Gel extraction for digestion product. We used FastGene Gel&PCR extraction kit(NipponGenetics)and got 50 ul of DNA solution.
August 17th
Colony PCR
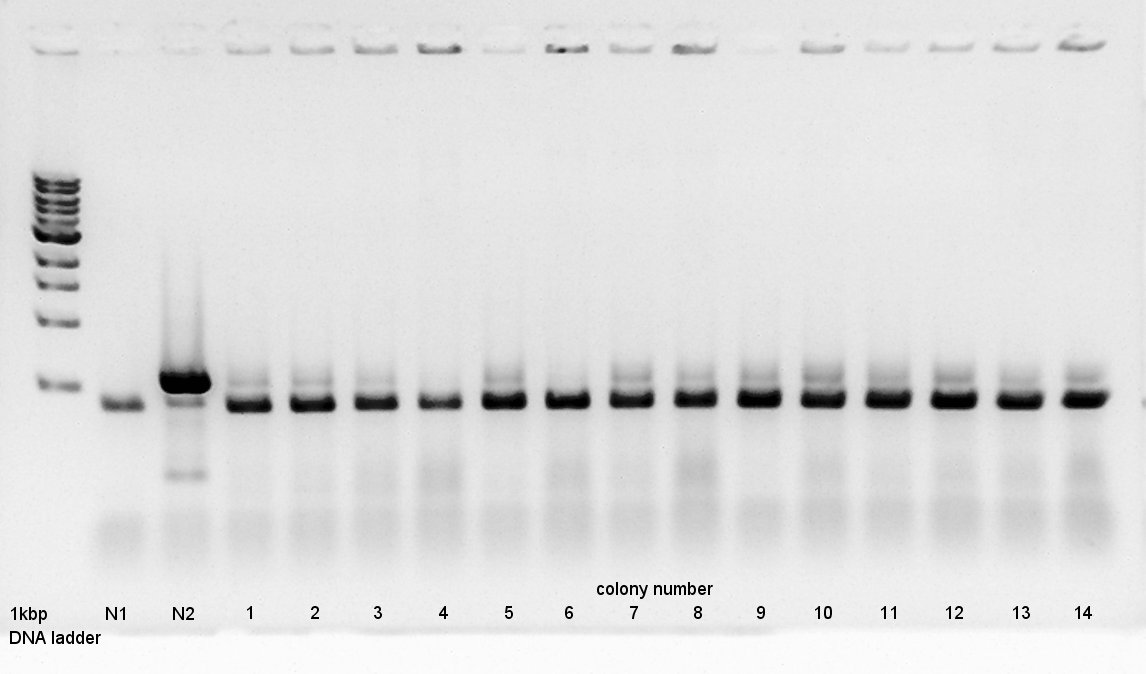
Colony PCR to confirm that whether the Ag43-dT was successfully ligated with pBAD-RBS on pSB1A3 or not.
| DNA solution | 4 ul (1 colony/10 ul DW) |
| Kapa-Taq(Taq polymerase) | 5 ul |
| Forward Primer(Ag43-f4 primer (50 pmol/ul)) | 0.5 ul |
| Reverse Primer(PS-R primer (50 pmol/ul)) | 0.5 ul |
| Total | 10 ul |
| Number | Degree | Second |
| 1 | 95 | 120 |
| 2 | 95 | 30 |
| 3 | 53 | 30 |
| 4 | 72 | 60 |
| 5 | 72 | 60 |
| 6 | 4 | HOLD |
Cycle:2~4 x 35
We used N1 (DW only) and N2 (Ag43-dt on pSB1K3) as controls. Desired product is about 800~1000bp.
From this result, N2 has about 500bp band.
We use to liquid culture, No. 7,10,12.
We cultured these DNA in E. coli solution, after added 200 ul LB, at 37C for 3 hrs.
Next step, we resuspended these 5 colonies and cultured (add 1800 ul LB and 2 ul Amp) for hours at 37C.
Transformation
Transformation of BBa_I13453 (pBAD on pSB1A3) into DH5α.
- Added 1 ul of plasmid DNA to 50 ul of thawed competent cells on ice.
- Incubated on ice for 30 min.
- Added 350 ul of LB.
- Prepared and Labeled two petri dishes with LBK.
- Plate 300 ul of the transformation onto first dish and spread.
- Added 450 ul of LB to 50 ul of the transformation and plated 300 ul of it onto second dish and spread.
- Incubated the plates at 37C for 19 hours.
Digestion
From sequencing results, we found that we failed to make pT7-RBS on pSB1K3.
So, we tried to make it again.
When the restriction enzyme digest the DNA, it is important for 3A assembry to be digested the target plasmid completely. So, we activated the restriction enzymes for 10 hours.
pT7 (BBa_I719005)
EcoRI/SpeI
| DNA solution (40 ng/ul) | 12.5 ul |
| EcoRI | 1 ul |
| SpeI | 1 ul |
| 10xH buffer | 2 ul |
| DW | 3.5 ul |
| Total | 20 ul |
RBS (BBa_B0034) XbaI/PstI
| DNA solution (40 ng/ul) | 12.5 ul |
| XbaI | 1 ul |
| PstI | 1 ul |
| 10xM buffer | 2 ul |
| DW | 3.5 ul |
| Total | 20 ul |
pSB1C3 EcoRI/PstI
| DNA solution (25 ng/ul) | 12 ul |
| EcoRI | 1 ul |
| PstI | 1 ul |
| 10xH buffer | 2 ul |
| DW | 4 ul |
| Total | 20 ul |
August 18th
Ethanol precipitation
Ethanol precipitation for three digestion products.
- Added 2 ul of NaoAc, 1.5 ul of glycogen and 50 ul of 100% ethanol.
- Centrifuged at 14000 rpm, 30 min at 4C.
- Removed supernatant and added 220 ul of 70% ethanol.
- Centrifuged at 15000 rpm, 15 min at 4C.
- Removed supernatant and dried out at room temperature, after that added 10 ul of DW.
Gel extraction
Before digestion of pBAD-RBS on pSB1A3 and Ag43-dT on pSB1AK3, it is necessary to refine these two DNA. We used FastGene Gel&PCR extraction kit(NipponGenetics)and got 20 ul of DNA solution from 10 ul of plasmid extraction products.
Digestion
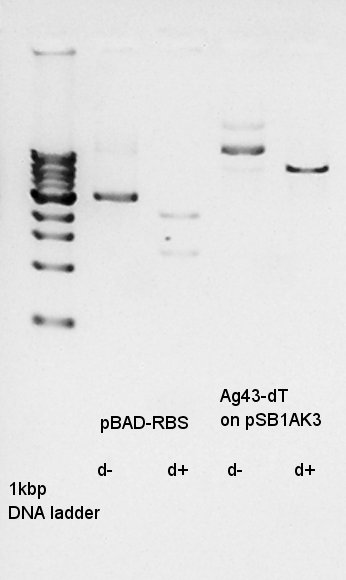
Digested pBAD-RBS on pSB1A3 as insert and Ag43-dT on pSB1AK3 as vector with EcoRI and SpeI to make a constract, pBAD-RBS-Ag43-dT on pSB1AK3.
pBAD-RBS
EcoRI/SpeI
| plasmid extraction product (20 ng/ul) | 20 ul |
| EcoRI | 1 ul |
| SpeI | 1 ul |
| 10xH buffer | 3 ul |
| DW | 5 ul |
| Total | 30 ul |
Ag43-dT on pSB1AK3
EcoRI/XbaI
| Gel extraction product (40 ng/ul) | 8 ul |
| EcoRI | 1 ul |
| XbaI | 1 ul |
| 10xH buffer | 3 ul |
| DW | 17 ul |
| Total | 30 ul |
Gel extraction
Gel extraction for digestion product. We used FastGene Gel&PCR extraction kit(NipponGenetics)and got 50 ul of DNA solution.
Ethanol precipitation
Ethanol precipitation for digestion products.
- Added 5 ul of NaoAc, 1.5 ul of glycogen and 125 ul of 100% ethanol.
- Centrifuged at 15000 rpm, 10 min at 4C.
- Removed supernatant and added 220 ul of 70% ethanol.
- Centrifuged at 15000 rpm, 5 min at 4C.
- Removed supernatant and dried out at room temperature, after that added 10 ul of DW.
Plasmid extraction
Plasmid extraction for colony number of No. 10 and 12 of pBAD-RBS-Ag43-dT. We used FastGene Plasmid Mini Kit(Nippon Genetics) and got 50 ul of DNA solutions.
Ligation
Ligation for pT7 and RBS as inserts and pSB1C3 as vector.
| pT7 (100 ng/ul) | 2 ul |
| Ag43-dT (100 ng/ul) | 2 ul |
| pSB1C3 (60 ng/ul) | 2 ul |
| Ligation Mighty Mix(TAKARA) | 6 ul |
| Total | 12 ul |
| Degree | Minute |
| 16 | 30 |
| 65 | 10 |
| 4 | Hold |
| pBAD-RBS (80 ng/ul) | 2 ul |
| Ag43-dT on pSB1AK3 (60 ng/ul) | 2 ul |
| DW | 1 ul |
| Ligation Mighty Mix(TAKARA) | 5 ul |
| Total | 10 ul |
| Degree | Minute |
| 16 | 30 |
| 65 | 10 |
| 4 | Hold |
August 19th
Transformation
Transformation of ligation product (two construct ligated at August 18) into DH5α.
pT7-RBS on pSB1C3
- Added 2 ul of plasmid DNA to 50 ul of thawed competent cells on ice.
- Incubated on ice for 30min.
- Added 350 ul of LB.
- Incubated at 37C for 2 hours.
- Prepared and Labeled two petri dishes with LBC.
- Plate 300 ul of the transformation onto first dish and spread.
- Added 450 ul of LB to 50 ul of the transformation and plated 300 ul of it onto second dish and spread.
- Incubated the plates at 37C for 18 hours.
pBAD-RBS-Ag43-dT on pSB1AK3
- Added 2 ul of plasmid DNA to 50 ul of thawed competent cells on ice.
- Incubated on ice for 30min.
- Added 350 ul of LBK.
- Incubated at 37C for 2 hours.
- Prepared and Labeled two petri dishes with LBK.
- Plate 300 ul of the transformation onto first dish and spread.
- Added 450 ul of LB to 50 ul of the transformation and plated 300 ul of it onto second dish and spread.
- Incubated the plates at 37C for 16 hours and 30 minutes.
PCR
PCR for pT7-RBS on pSB1K3. By sequencing reaction, we found the No. 10 plasmid is not pT7-RBS on pSB1K3. We checked the other plasmid is the same or not.
| DNA solution | 1 ul |
| KOD-Plus-NEO(Taq polymerase) | 1 ul |
| dNTP | 5 ul |
| MgSO4 | 3 ul |
| KOD-Plus-NEO Buffer | 5 ul |
| Forward Primer(EX-Forward primer : 10 uM) | 1 ul |
| Reverse Primer(PS-Reverse primer : 10 uM) | 1 ul |
| DW | 33 ul |
| Total | 50 ul |
| Number | Degree | Second |
| 1 | 94 | 120 |
| 2 | 98 | 10 |
| 3 | 68 | 30 |
| 4 | 4 | HOLD |
Cycle:2~3 x 45
No. 4 plasmid has independent bands. However, if it is pT7-RBS on pSB1K3, it has about 60~70bp band.
 "
"