Team:HokkaidoU Japan/Notebook/aggregation Week 6
From 2012.igem.org
Contents |
August 6th
Ethanol precipitation
Ethanol precipitation for digestion and gel extraction product.
- Added 5 ul of NaoAc, 1.5 ul of glycogen and 125 ul of 100% ethanol.
- Centrifuged in 15000 rpm, 10 min at 4C.
- Remove supernatant and added 220 ul of 70% ethanol.
- Centrifuged in 15000 rpm, 10 min at 4C.
- Remove supernatant and air drying in room temperature then added 10 ul of DW.
Ligation
We ligated pT7-RBS on pSB1K3 (digested Spe1) as vector and Ag43-dT (digested Spe1 and Xba1 and HindIII) as insert.
| pT7-RBS | 1 ul |
| Ag43-dT | 2 ul |
| DW | 2 ul |
| Ligation Mighty Mix(TAKARA) | 5 ul |
| Total | 10 ul |
Ligation reaction time was written below.
| Degree | Minute |
| 16 | 30 |
| 65 | 10 |
| 4 | Hold |
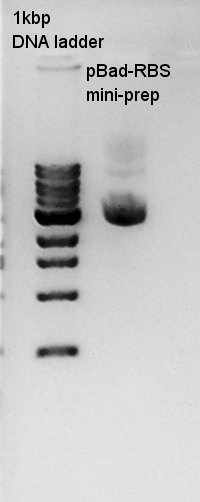
mini-prep
mini-prep of pBad-RBS. We used FastGene Plasmid Mini Kit(Nippon Genetics) and got 50 ul of DNA solutions.
transformation
Transformation of plasmid DNA (pT7-RBS-Ag43-dT on pSB1K3)
in E. coli(DH5α).
- Added 2 ul of plasmid DNA to 50 ul of thawed competent cells on ice.
- Incubated on ice for 30 min.
- Added 600 ul of LB then incubated the cells for 2 hours at 37C.
- Prepared and Labeled two LBK plates.
- Plated 300 ul of the culture onto first dish and spread.
- Added 900 ul of LB to 100 ul of the culture and plated 300 ul of it onto second dish and spread.
- Incubated them at 37C for 16 hours.
Ethanol precipitation
Ethanol precipitation for mini-prep product (pBad-RBS). Because the refine of mini-prep product is not enough.
We use 15 ul DNA solution.
- Added 1.5 ul of NaoAc(3 M), 1.5 ul of glycogen and 38 ul of 100% ethanol.
- Centrifuged in 15000 rpm, 10 min at 4C.
- Remove supernatant and added 220 ul of 70% ethanol.
- Centrifuged in 15000 rpm, 5 min at 4C.
- Remove supernatant and air drying in room temperature then added 10 ul of DW.
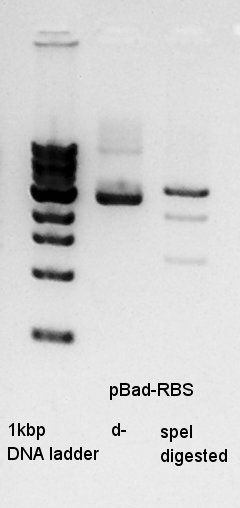
digestion
digestion of ethanol precipitation product(pBad-RBS).
| DNA solution | 3 ul |
| SpeI | 1 ul |
| 10xM buffer | 1 ul |
| DW | 5 ul |
| Total | 10 ul |
In this result, speI digested DNA completely.
So I think that the pT7-RBS on pSB1K3 DNA solution is not pure.
August 7th
Gel extraction
Gel extraction for digestion product. We used FastGene Gel&PCR extraction kit(NipponGenetics)and got 50 ul of DNA solution.
Ethanol precipitation
Ethanol precipitation for digestion and gel extraction product.
- Added 5 ul of NaoAc, 1.5 ul of glycogen and 125 ul of 100% ethanol.
- Centrifuged in 15000 rpm, 10 min at 4C.
- Remove supernatant and added 220 ul of 70% ethanol.
- Centrifuged in 15000 rpm, 10 min at 4C.
- Remove supernatant and air drying in room temperature then added 10 ul of DW.
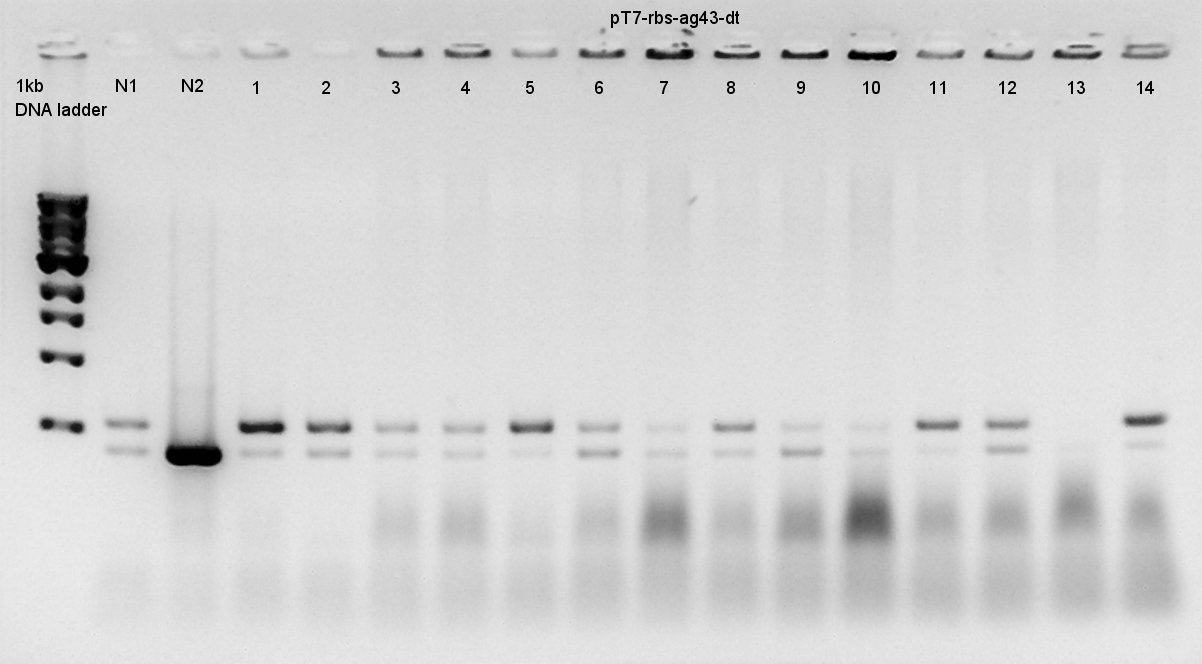
Colony PCR
Colony PCR to confirm that whether the Ag43-dT was successfully ligated with pT7-RBS on pSB1K3 or not.
| DNA solution | 4 ul (1colony/10 ul DW) |
| Kapa-Taq(Taq polymerase) | 5 ul |
| Forward Primer(Ag43-f4 primer) | 0.5 ul |
| Reverse Primer(PS-R primer) | 0.5 ul |
| Total | 10 ul |
| Number | Degree | Second |
| 1 | 95 | 120 |
| 2 | 95 | 30 |
| 3 | 53 | 30 |
| 4 | 72 | 60 |
| 5 | 72 | 60 |
| 6 | 4 | HOLD |
Cycle:2~4 x 35
We used N1 (DW only) and N2 (Ag43 on pSB1C3 (K346007)) as controls. Desired product is about 500~600bp.
We thought that colonies of No. 1 and 2 and 5 and 11 and 14 have deep band. Next step, we resuspended these 5 colonies and cultured (add 1700 ul LB and 2 ul Amp) for 15 hours in 37C.
August 8th
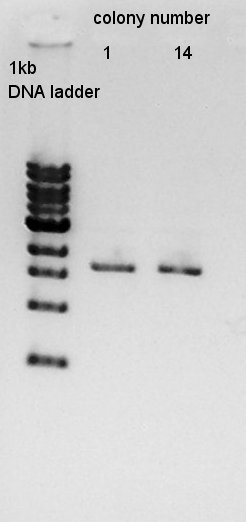
mini-prep
mini-prep of colony No. 1 and 14. We used FastGene Plasmid Mini Kit(Nippon Genetics) and got 50 ul of DNA solutions.
And then, we did mini-prep for colony No. 2,5,11. The results of them are the same.
Sequencing
Retry for sequencing..
| DNA | primer | ||||
| Ag43 mini-prep product | 100bp-up forward primer, | ag43-f1, | ag43-f2, | ag43-f3, | ag43-f4 |
| K542009 | 100bp-up forward primer, | ag43-f1, | ag43-f2, | ag43-f3, | ag43-f4 |
| pT7-RBS on pSB1K3 | 100bp-up forward primer | ||||
| pT7-RBS on pSB1C3 | 100bp-up forward primer | ||||
| Ag43-dT on pSB1AK3 | 100bp-up forward primer, | ag43-f1, | ag43-f2, | ag43-f3, | ag43-f4 |
| Ag43-dT on pSB1T3 | 100bp-up forward primer, | ag43-f1, | ag43-f2, | ag43-f3, | ag43-f4 |
| pT7-RBS on pSB1K3 | 100bp-up forward primer |
Sequencing PCR
| template DNA | 1 ul |
| Ready Reaction Premix | 1 ul |
| 5x Sequencing Buffer | 1.5 ul |
| H2O | 5 ul |
| Primer(1pmol/ul) | 1.5 ul |
| Total | 10 ul |
| Number | Degree | Second |
| 1 | 96 | 10 |
| 2 | 50 | 5 |
| 3 | 60 | 240 |
| 4 | 4 | HOLD |
Cycle:1~3 x 25
To purify the PCR product, we did ethanol precipitation.
Ethanol precipitation in our sequencing protocol
- Added 10 ul of H2O, 2 ul of NaoAc, 1 ul of glycogen and 50 ul of 100% ethanol.
- Centrifuged in 15000 rpm, 15 min at 26C.
- Remove supernatant and added 100ul of 70% ethanol.
- Centrifuged in 15000 rpm, 5 min at 26C.
- Remove supernatant and air drying in room temperature then added 10 ul of Hi-Di.
Then run a sequencing machine (ByoDye Terminator v3.1 Cycle Sequencing Kit)
August 9th
Ligation
We ligated pT7-RBS on pSB1K3 (digested Spe1) as vector and Ag43-dT (digested Spe1 and Xba1 and HindIII) as insert.
We did ligation (pT7-RBS on pSB1K3 and Ag43-dT) and transformation it, at August 6th.
However, we could not get the target DNA (pT7-RBS-Ag43-dT on pSB1K3 ).
So, we did ligation by using more Insert DNA part.
| pT7-RBS | 1 ul |
| Ag43-dT | 4 ul |
| Ligation Mighty Mix(TAKARA) | 5 ul |
| Total | 10 ul |
Ligation reaction time was written below.
| Degree | Minute |
| 16 | 30 |
| 65 | 10 |
| 4 | Hold |
 "
"