Team:HokkaidoU Japan/Notebook/aggregation Week 10
From 2012.igem.org
| Line 4: | Line 4: | ||
<!-- DO NOT EDIT OVER THIS LINE @iTakeshi --> | <!-- DO NOT EDIT OVER THIS LINE @iTakeshi --> | ||
<div class="hokkaidou-notebook-daily"> | <div class="hokkaidou-notebook-daily"> | ||
| - | ==September 3rd== | + | ===September 3rd=== |
| - | <div> | + | <div class="hokkaidou-section"> |
| - | + | ====Colony PCR==== | |
| - | ==Colony PCR== | + | |
| - | + | ||
Colony PCR to confirm that whether the pBAD-RBS-Ag43-dT was successfully ligated with pSB1C3 or not. | Colony PCR to confirm that whether the pBAD-RBS-Ag43-dT was successfully ligated with pSB1C3 or not. | ||
| - | |||
{|class="hokkaidou-table-pcr-reagent" | {|class="hokkaidou-table-pcr-reagent" | ||
| Line 68: | Line 65: | ||
We used N1 (DW only) and N2 (pBAD-RBS-Ag43-dT on pSB1Ak3) as controls. | We used N1 (DW only) and N2 (pBAD-RBS-Ag43-dT on pSB1Ak3) as controls. | ||
Desired product is about 600bp. | Desired product is about 600bp. | ||
| - | + | ||
[[image:HokkaidoU2012_120903_ag43s_psb1c3.jpg|thumb|Colony PCR result]] | [[image:HokkaidoU2012_120903_ag43s_psb1c3.jpg|thumb|Colony PCR result]] | ||
| - | ==Colony PCR of eCFP-RBS-pSB1A2== | + | ====Colony PCR of eCFP-RBS-pSB1A2==== |
| - | + | ||
| - | + | ||
| - | + | ||
{|class="hokkaidou-table-pcr-reagent" | {|class="hokkaidou-table-pcr-reagent" | ||
|- | |- | ||
| Line 130: | Line 124: | ||
[[image:HokkaidoU2012 120903 colop eCFP-RBS-pSB1A2 EX-F PS-R (2).jpg|thumb|Colony PCR result]] | [[image:HokkaidoU2012 120903 colop eCFP-RBS-pSB1A2 EX-F PS-R (2).jpg|thumb|Colony PCR result]] | ||
| - | |||
We confirmed that about 70% of ligated DNA formed our desired construct. We selected No. 2 and 5 colony for incubation and store No. 7 and 8 colony mixture at 4C. | We confirmed that about 70% of ligated DNA formed our desired construct. We selected No. 2 and 5 colony for incubation and store No. 7 and 8 colony mixture at 4C. | ||
| - | |||
| - | ==Incubation of eCFP-RBS-pSB1A2 for plasmid extraction== | + | ====Incubation of eCFP-RBS-pSB1A2 for plasmid extraction==== |
| - | + | ||
#Prepared 2 ml LBA into culture tubes. | #Prepared 2 ml LBA into culture tubes. | ||
#Re-suspended 2 colony mixture (No. 2 and No. 5 respectively). | #Re-suspended 2 colony mixture (No. 2 and No. 5 respectively). | ||
#Incubated at 37C for 17 hrs. | #Incubated at 37C for 17 hrs. | ||
| - | |||
| - | + | ====Estimation of concentration of eCFP-RBS-pSB1A2 and pBAD-RBS-pSB1A2==== | |
| - | ==Estimation of concentration of eCFP-RBS-pSB1A2 and pBAD-RBS-pSB1A2== | + | |
| - | + | ||
No. 2,5 means the colony number of colony PCR of eCFP-RBS-pSB1A2. | No. 2,5 means the colony number of colony PCR of eCFP-RBS-pSB1A2. | ||
| - | |||
[[image:HokkaidoU2012 120903 mini-prep eCFP-RBS-1A2No.2 sameNo.jpg|thumb|electrophoresis result]] | [[image:HokkaidoU2012 120903 mini-prep eCFP-RBS-1A2No.2 sameNo.jpg|thumb|electrophoresis result]] | ||
| - | |||
We estimated the concentration of eCFP-RBS-pSB1A2 is 30 ng/ul and pBAD-RBS-pSB1A2 is 50 ng/ul. | We estimated the concentration of eCFP-RBS-pSB1A2 is 30 ng/ul and pBAD-RBS-pSB1A2 is 50 ng/ul. | ||
| - | |||
| - | |||
| - | |||
| - | ==Digestion of eCFP-RBS-pSB1A2 and pBAD-RBS-pSB1A2== | + | ====Digestion of eCFP-RBS-pSB1A2 and pBAD-RBS-pSB1A2==== |
| - | + | ||
To make a construct of pBAD-RBS-eCFP-RBS-pSB1A2, we digested eCFP-RBS-pSB1A2 with XbaI & PstI and pBAD-RBS-pSB1A2 with SpeI and PstI. And we digested eCFP-pSB1A2 with XbaI and SpeI as a control for confirmation of the ability to digest. | To make a construct of pBAD-RBS-eCFP-RBS-pSB1A2, we digested eCFP-RBS-pSB1A2 with XbaI & PstI and pBAD-RBS-pSB1A2 with SpeI and PstI. And we digested eCFP-pSB1A2 with XbaI and SpeI as a control for confirmation of the ability to digest. | ||
| - | + | <br /> | |
Insert (eCFP-RBS-pSB1A2) | Insert (eCFP-RBS-pSB1A2) | ||
{|class="hokkaidou-table-digestion" | {|class="hokkaidou-table-digestion" | ||
| Line 181: | Line 163: | ||
|30 ul | |30 ul | ||
|} | |} | ||
| - | |||
| - | |||
| Line 229: | Line 209: | ||
|20 ul | |20 ul | ||
|} | |} | ||
| - | |||
| Line 250: | Line 229: | ||
|HOLD | |HOLD | ||
|} | |} | ||
| - | |||
[[image:HokkaidoU2012 120903 Digesiton eCFP-RBS(XP) pBAD-RBS-pSB1A2(SP) Control(XS).jpg|thumb|digestion result]] | [[image:HokkaidoU2012 120903 Digesiton eCFP-RBS(XP) pBAD-RBS-pSB1A2(SP) Control(XS).jpg|thumb|digestion result]] | ||
| - | |||
From this image, we confirmed that DNA were digested into fragments and all of restriction enzyme worked. | From this image, we confirmed that DNA were digested into fragments and all of restriction enzyme worked. | ||
| - | |||
</div></div> | </div></div> | ||
| - | |||
<div class="hokkaidou-notebook-daily"> | <div class="hokkaidou-notebook-daily"> | ||
| - | ==September 4th== | + | ===September 4th=== |
| - | <div> | + | <div class="hokkaidou-section"> |
| - | ==Plasmid extraction== | + | ====Plasmid extraction==== |
| - | + | ||
Plasmid extraction of pBAD-RBS-Ag43-dT on pSB1C3. | Plasmid extraction of pBAD-RBS-Ag43-dT on pSB1C3. | ||
We used plasmid extraction kit of Nippon genetics: FastGene Plasmid mini kit and finally we eluted the DNA with 50 ul of elution buffer. | We used plasmid extraction kit of Nippon genetics: FastGene Plasmid mini kit and finally we eluted the DNA with 50 ul of elution buffer. | ||
| - | |||
[[image:HokkaidoU2012_120903_ag43_comple_psb1c3.jpg|thumb|Plasmid extraction result]] | [[image:HokkaidoU2012_120903_ag43_comple_psb1c3.jpg|thumb|Plasmid extraction result]] | ||
| - | ==Ethanol precipitation of digestion products (eCFP-RBS and pBAD-RBS-pSB1A2) and estimation of concentration== | + | ====Ethanol precipitation of digestion products (eCFP-RBS and pBAD-RBS-pSB1A2) and estimation of concentration==== |
| - | + | ||
#Added 5 ul of NaoAc, 1.5 ul of glycogen and 125 ul of 100% ethanol. | #Added 5 ul of NaoAc, 1.5 ul of glycogen and 125 ul of 100% ethanol. | ||
#Centrifuged at 15000 rpm, 15 min at 4C. | #Centrifuged at 15000 rpm, 15 min at 4C. | ||
| Line 278: | Line 250: | ||
#Centrifuged at 15000 rpm, 10 min at 4C. | #Centrifuged at 15000 rpm, 10 min at 4C. | ||
#Removed supernatant and dried out at room temperature, after that added 10 ul of DW. | #Removed supernatant and dried out at room temperature, after that added 10 ul of DW. | ||
| - | |||
[[image:HokkaidoU2012 120904 Ethapre Insert Vector.jpg|thumb|ethanol precipitation result]] | [[image:HokkaidoU2012 120904 Ethapre Insert Vector.jpg|thumb|ethanol precipitation result]] | ||
| - | |||
We estimated the concentration of ethanol presipitation products.The concentration of Insert DNA solution is about 20 ng/ul and Vector DNA solution is about 50 ng/ul. | We estimated the concentration of ethanol presipitation products.The concentration of Insert DNA solution is about 20 ng/ul and Vector DNA solution is about 50 ng/ul. | ||
| - | |||
| - | ==Ligation of eCFP-RBS and pBAD-RBS-pSB1A2== | + | ====Ligation of eCFP-RBS and pBAD-RBS-pSB1A2==== |
| - | + | ||
{|class="hokkaidou-table-ligation" | {|class="hokkaidou-table-ligation" | ||
|- | |- | ||
| Line 323: | Line 291: | ||
|} | |} | ||
| - | + | ====Transformation of pBAD-RBS-eCFP-RBS-pSB1A2==== | |
| - | + | ||
| - | ==Transformation of pBAD-RBS-eCFP-RBS-pSB1A2== | + | |
| - | + | ||
#Mixed 2 ul pBAD-RBS-eCFP-RBS-pSB1A2 ligation product to 50 ul of thawed competent cells on ice. | #Mixed 2 ul pBAD-RBS-eCFP-RBS-pSB1A2 ligation product to 50 ul of thawed competent cells on ice. | ||
#Incubated on ice for 30 min. | #Incubated on ice for 30 min. | ||
| Line 334: | Line 299: | ||
#Mixed 450 ul of LB to 50 ul of the culture and plated 300 ul of it onto second dish and spread. | #Mixed 450 ul of LB to 50 ul of the culture and plated 300 ul of it onto second dish and spread. | ||
#Incubated the plates at 37C for 25 hours. | #Incubated the plates at 37C for 25 hours. | ||
| - | |||
| - | + | ====Digestion of ptet-RBS-eYFP-dT-pSB1A2 and pSTV28==== | |
| - | ==Digestion of ptet-RBS-eYFP-dT-pSB1A2 and pSTV28== | + | |
| - | + | ||
To make a construct of ptet-RBS-eYFP-dT-pSTV28, we digested ptet-RBS-eYFP-dT-pSB1A2 with EcoRI and PstI, and pSTV28 with EcoRI and PstI. And we digested Ag43-dT-pSB1AK3 by HindIII. | To make a construct of ptet-RBS-eYFP-dT-pSTV28, we digested ptet-RBS-eYFP-dT-pSB1A2 with EcoRI and PstI, and pSTV28 with EcoRI and PstI. And we digested Ag43-dT-pSB1AK3 by HindIII. | ||
| - | + | <br /> | |
Insert (ptet-RBS-eYFP-dT-pSB1A2) | Insert (ptet-RBS-eYFP-dT-pSB1A2) | ||
{|class="hokkaidou-table-digestion" | {|class="hokkaidou-table-digestion" | ||
| Line 362: | Line 324: | ||
|30 ul | |30 ul | ||
|} | |} | ||
| - | |||
| - | |||
| Line 407: | Line 367: | ||
|50 ul | |50 ul | ||
|} | |} | ||
| - | |||
| Line 428: | Line 387: | ||
|HOLD | |HOLD | ||
|} | |} | ||
| - | |||
| - | |||
[[image:HokkaidoU2012 120906 Digestion Insert Vector Ag43-dT-1AK3(HindIII).jpg|thumb|digestion result]] | [[image:HokkaidoU2012 120906 Digestion Insert Vector Ag43-dT-1AK3(HindIII).jpg|thumb|digestion result]] | ||
| - | |||
We confirmed that ptet-RBS-eYFP-dT-pSB1A2 was partially digested and pSTV28 would be successfully digested we think. But we couldn't confirm whether Ag43-dT-pSB1AK3 was digested or not. | We confirmed that ptet-RBS-eYFP-dT-pSB1A2 was partially digested and pSTV28 would be successfully digested we think. But we couldn't confirm whether Ag43-dT-pSB1AK3 was digested or not. | ||
| Line 439: | Line 395: | ||
<div class="hokkaidou-notebook-daily"> | <div class="hokkaidou-notebook-daily"> | ||
| - | ==September 5th== | + | ===September 5th=== |
| - | <div> | + | <div class="hokkaidou-section"> |
| - | ==Digestion== | + | ====Digestion==== |
| - | + | ||
We digested plasmid extraction products (pBAD-RBS-Ag43-dT on pSB1AK3) by restriction enzyme. | We digested plasmid extraction products (pBAD-RBS-Ag43-dT on pSB1AK3) by restriction enzyme. | ||
Because I'd like to check the size of insert and vector of getting plasmid. | Because I'd like to check the size of insert and vector of getting plasmid. | ||
| - | |||
No. 1 | No. 1 | ||
| Line 567: | Line 521: | ||
|HOLD | |HOLD | ||
|} | |} | ||
| - | |||
| - | |||
[[image:HokkaidoU2012_120905_ag43_comp_dig.jpg|thumb|Digestion resulsts]] | [[image:HokkaidoU2012_120905_ag43_comp_dig.jpg|thumb|Digestion resulsts]] | ||
| - | |||
</div></div> | </div></div> | ||
| - | |||
<div class="hokkaidou-notebook-daily"> | <div class="hokkaidou-notebook-daily"> | ||
| - | + | ===September 6th=== | |
| - | ==September 6th== | + | <div class="hokkaidou-section"> |
| - | <div> | + | ====Ag43 expressing==== |
| - | ==Ag43 expressing== | + | |
| - | + | ||
We usually used plastic tube for incubating. | We usually used plastic tube for incubating. | ||
| Line 590: | Line 538: | ||
We estimated the reason is attachment to hydrophobic materials. | We estimated the reason is attachment to hydrophobic materials. | ||
We incubated in 1 ml of LBA (one contained 1% of L-arabinose, another did not contain L-arabinose) at 37C in glass tubes. | We incubated in 1 ml of LBA (one contained 1% of L-arabinose, another did not contain L-arabinose) at 37C in glass tubes. | ||
| - | |||
| - | ==Ethanol precipitation of ptet-RBS-eYFP-dT-pSB1A2 and pSTV28== | + | ====Ethanol precipitation of ptet-RBS-eYFP-dT-pSB1A2 and pSTV28==== |
| - | + | ||
#Added 5 ul of NaoAc, 1.5 ul of glycogen and 125 ul of 100% ethanol. | #Added 5 ul of NaoAc, 1.5 ul of glycogen and 125 ul of 100% ethanol. | ||
#Centrifuged at 14000 rpm, 30 min at 4C. | #Centrifuged at 14000 rpm, 30 min at 4C. | ||
| Line 599: | Line 545: | ||
#Centrifuged at 15000 rpm, 15 min at 4C. | #Centrifuged at 15000 rpm, 15 min at 4C. | ||
#Removed supernatant and dried out at room temperature, after that added 10 ul of DW. | #Removed supernatant and dried out at room temperature, after that added 10 ul of DW. | ||
| - | |||
[[image:HokkaidoU2012 120906 Ethapre Insert Vector.jpg|thumb|ethanol precipitation result]] | [[image:HokkaidoU2012 120906 Ethapre Insert Vector.jpg|thumb|ethanol precipitation result]] | ||
We confirmed that the concentration of Insert DNA solution is 10~20 ng/ul and Vector DNA solution is 30~40 ng/ul. | We confirmed that the concentration of Insert DNA solution is 10~20 ng/ul and Vector DNA solution is 30~40 ng/ul. | ||
| - | |||
| - | ==Ligation of ptet-RBS-eYFP-dT-pSB1A2 and pSTV28== | + | ====Ligation of ptet-RBS-eYFP-dT-pSB1A2 and pSTV28==== |
| - | + | ||
{|class="hokkaidou-table-ligation" | {|class="hokkaidou-table-ligation" | ||
|- | |- | ||
| Line 643: | Line 586: | ||
|} | |} | ||
| - | |||
| - | ==Transformation of ptet-RBS-eYFP-dT-pSTV28== | + | |
| - | + | ====Transformation of ptet-RBS-eYFP-dT-pSTV28==== | |
#Mixed 2 ul ptet-RBS-eYFP-dT-pSTV28 ligation product to 50 ul of thawed competent cells on ice. | #Mixed 2 ul ptet-RBS-eYFP-dT-pSTV28 ligation product to 50 ul of thawed competent cells on ice. | ||
#Incubated on ice for 30 min. | #Incubated on ice for 30 min. | ||
| Line 655: | Line 597: | ||
#Mixed 450 ul of LB to 50 ul of the culture and plated 300 ul of it onto second dish and spread. | #Mixed 450 ul of LB to 50 ul of the culture and plated 300 ul of it onto second dish and spread. | ||
#Incubated the plates at 37C for 19 hrs. | #Incubated the plates at 37C for 19 hrs. | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| + | ====Colony PCR of pBAD-RBS-eCFP-RBS-pSB1A2==== | ||
{|class="hokkaidou-table-pcr-reagent" | {|class="hokkaidou-table-pcr-reagent" | ||
|- | |- | ||
| Line 717: | Line 654: | ||
[[image:HokkaidoU2012 120906 colop pBAD-RBS-eCFP-RBS-pSB1A2.jpg|thumb|Colony PCR result]] | [[image:HokkaidoU2012 120906 colop pBAD-RBS-eCFP-RBS-pSB1A2.jpg|thumb|Colony PCR result]] | ||
| - | |||
We thought that almost all of ligated DNA successfully ligated to our desired construct. We selected No. 1 colony for incubation and store No. 2,3 and 4 colony liquid medium at 4C. | We thought that almost all of ligated DNA successfully ligated to our desired construct. We selected No. 1 colony for incubation and store No. 2,3 and 4 colony liquid medium at 4C. | ||
| - | |||
| - | ==Incubation of pBAD-RBS-eCFP-RBS-pSB1A2 for plasmid extraction== | + | ====Incubation of pBAD-RBS-eCFP-RBS-pSB1A2 for plasmid extraction==== |
| - | + | ||
#Prepared 2 ml LBA into culture tubes. | #Prepared 2 ml LBA into culture tubes. | ||
#Re-suspended 1 colony mixture. | #Re-suspended 1 colony mixture. | ||
#Incubated at 37C for 18 hrs. | #Incubated at 37C for 18 hrs. | ||
| - | |||
| - | ==Transformation of ptet-RBS-eYFP-dT-pSTV28 and Test to confirm appropriate antibiotic amount for screening of pSTV28== | + | ====Transformation of ptet-RBS-eYFP-dT-pSTV28 and Test to confirm appropriate antibiotic amount for screening of pSTV28==== |
| - | + | ||
To confirm how amount of chloramphenicol is needed for screening pSTV28 (Low copy plasmid) , we plated transformed cells on the special LBC which contains half amount of chloramphenicol we usually add. Now we call this LBC plate medium as LB1/2C. | To confirm how amount of chloramphenicol is needed for screening pSTV28 (Low copy plasmid) , we plated transformed cells on the special LBC which contains half amount of chloramphenicol we usually add. Now we call this LBC plate medium as LB1/2C. | ||
#Mixed 2 ul ptet-RBS-eYFP-dT-pSTV28 ligation product to 50 ul of thawed competent cells on ice. | #Mixed 2 ul ptet-RBS-eYFP-dT-pSTV28 ligation product to 50 ul of thawed competent cells on ice. | ||
| Line 740: | Line 672: | ||
#Mixed 450 ul of LB to 50 ul of the culture and plated 300 ul of it onto second dish and spread. | #Mixed 450 ul of LB to 50 ul of the culture and plated 300 ul of it onto second dish and spread. | ||
#Incubated the plates at 37C for 19 hrs. | #Incubated the plates at 37C for 19 hrs. | ||
| - | + | ||
</div></div> | </div></div> | ||
| - | |||
<div class="hokkaidou-notebook-daily"> | <div class="hokkaidou-notebook-daily"> | ||
| - | ==September 7th== | + | ===September 7th=== |
| - | <div> | + | <div class="hokkaidou-section"> |
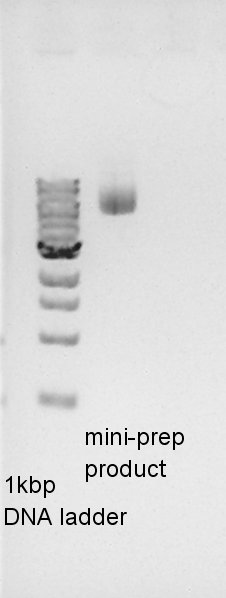
| - | ==Estimation of concentration of pBAD-RBS-eCFP-RBS-pSB1A2== | + | ====Estimation of concentration of pBAD-RBS-eCFP-RBS-pSB1A2==== |
| - | + | ||
| - | + | ||
[[image:HokkaidoU2012 120907 mini-prep pBAD-RBS-eCFP-RBS-pSB1A2.jpg|thumb|electrophoresis result]] | [[image:HokkaidoU2012 120907 mini-prep pBAD-RBS-eCFP-RBS-pSB1A2.jpg|thumb|electrophoresis result]] | ||
| - | |||
| - | |||
We estimated the concentration of pBAD-RBS-eCFP-RBS-pSB1A2 is about 30 ng/ul. | We estimated the concentration of pBAD-RBS-eCFP-RBS-pSB1A2 is about 30 ng/ul. | ||
| - | |||
| - | ==Incubation of ptet-RBS-eYFP-dT-pSTV28 for plasmid extraction== | + | ====Incubation of ptet-RBS-eYFP-dT-pSTV28 for plasmid extraction==== |
| - | + | ||
#Prepared 5~6 ml LBC into culture tubes. | #Prepared 5~6 ml LBC into culture tubes. | ||
#Re-suspended 2 colony. | #Re-suspended 2 colony. | ||
| Line 764: | Line 689: | ||
[[image:HokkaidoU2012 120907 mini-prep(Low-copy) ptet-RBS-eYFP-dT-pSTV28.jpg|thumb|Plasmid extraction result]] | [[image:HokkaidoU2012 120907 mini-prep(Low-copy) ptet-RBS-eYFP-dT-pSTV28.jpg|thumb|Plasmid extraction result]] | ||
| - | |||
</div></div> | </div></div> | ||
| + | |||
<!-- DO NOT EDIT UNDER THIS LINE @iTakeshi --> | <!-- DO NOT EDIT UNDER THIS LINE @iTakeshi --> | ||
</div> | </div> | ||
<br style="line-height: 0; clear: both;" /> | <br style="line-height: 0; clear: both;" /> | ||
{{Team:HokkaidoU_Japan/footer}} | {{Team:HokkaidoU_Japan/footer}} | ||
Revision as of 02:48, 27 September 2012
September 3rd
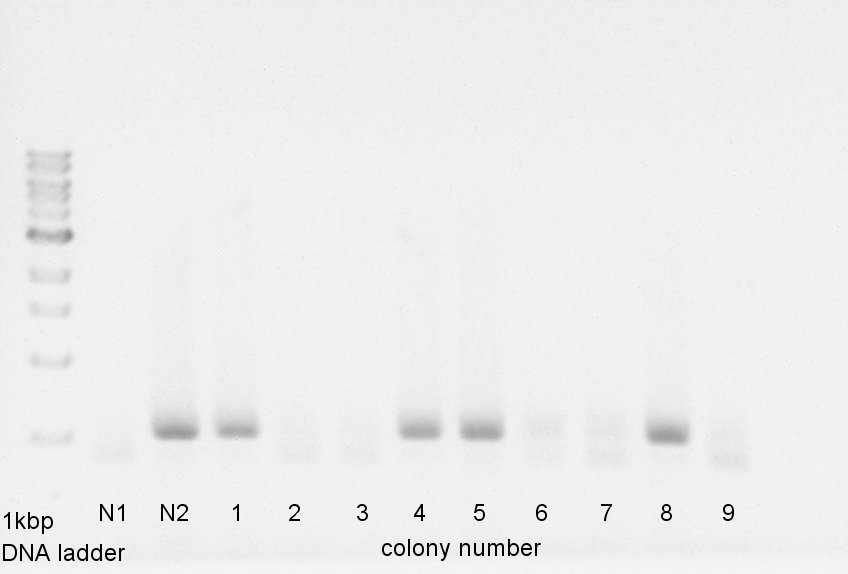
Colony PCR
Colony PCR to confirm that whether the pBAD-RBS-Ag43-dT was successfully ligated with pSB1C3 or not.
| DNA solution | 4 ul |
| Kapa-Taq(Taq polymerase) | 10 ul |
| Forward Primer(Ag43-f4) | 1 ul |
| Reverse Primer(PS-Reverse) | 1 ul |
| DW | 4 ul |
| Total | 20 ul |
| Number | Degree | Second |
| 1 | 95 | 120 |
| 2 | 95 | 30 |
| 3 | 53.2 | 30 |
| 4 | 72 | 60 |
| 5 | 72 | 60 |
| 6 | 4 | HOLD |
Cycle:2~4 x 35
We used N1 (DW only) and N2 (pBAD-RBS-Ag43-dT on pSB1Ak3) as controls. Desired product is about 600bp.
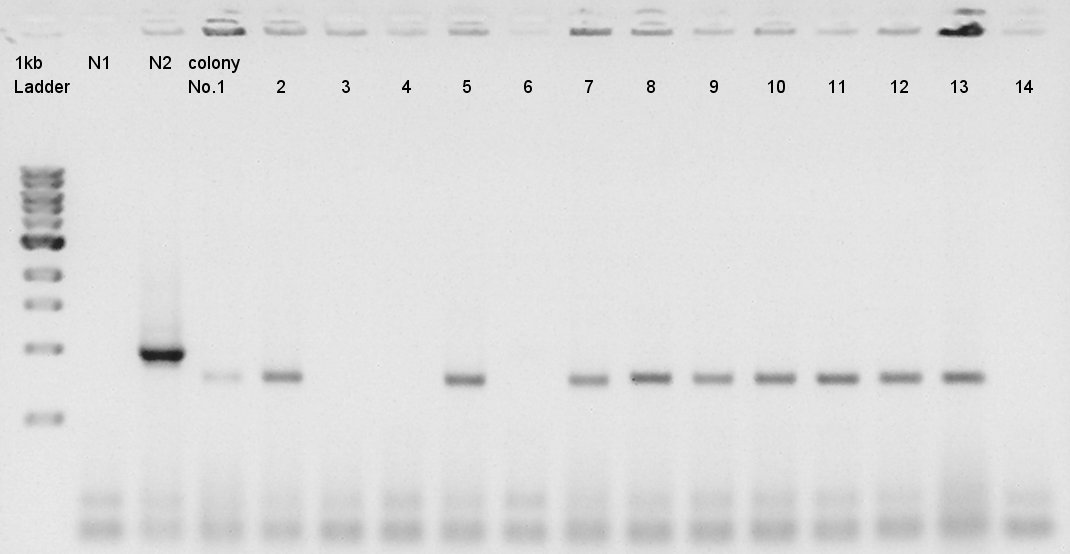
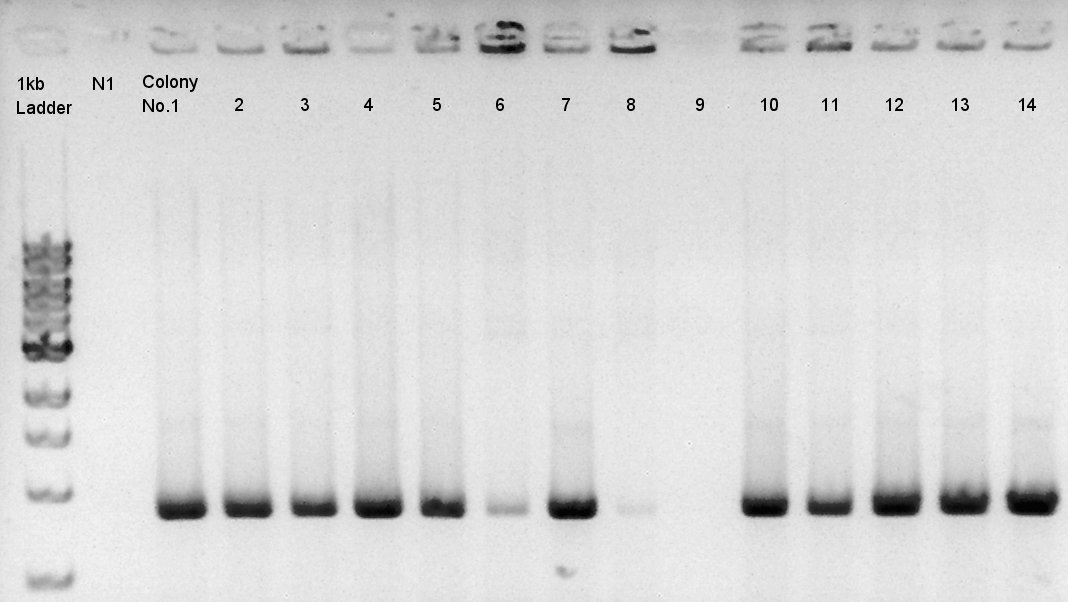
Colony PCR of eCFP-RBS-pSB1A2
| DNA solution | 4 ul |
| Kapa-Taq(Taq polymerase) | 5 ul |
| Forward Primer(EX-F primer) | 0.5 ul |
| Reverse Primer(PS-R down primer) | 0.5 ul |
| Total | 10 ul |
| Number | Degree | Second |
| 1 | 95 | 120 |
| 2 | 95 | 30 |
| 3 | 68.9 | 30 |
| 4 | 72 | 60 |
| 5 | 72 | 60 |
| 6 | 4 | HOLD |
Cycle:2~4 x 35
We used N1 (DW only) and N2 (ptetR-RBS-eCFP-dT-pSB1A2) as controls. Desired product is about 776bp.
We confirmed that about 70% of ligated DNA formed our desired construct. We selected No. 2 and 5 colony for incubation and store No. 7 and 8 colony mixture at 4C.
Incubation of eCFP-RBS-pSB1A2 for plasmid extraction
- Prepared 2 ml LBA into culture tubes.
- Re-suspended 2 colony mixture (No. 2 and No. 5 respectively).
- Incubated at 37C for 17 hrs.
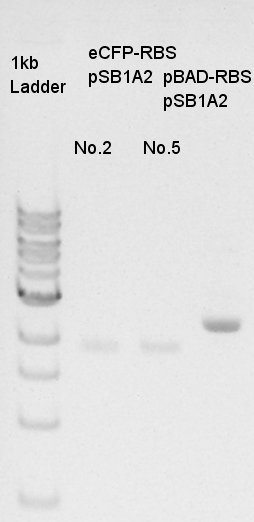
Estimation of concentration of eCFP-RBS-pSB1A2 and pBAD-RBS-pSB1A2
No. 2,5 means the colony number of colony PCR of eCFP-RBS-pSB1A2.
We estimated the concentration of eCFP-RBS-pSB1A2 is 30 ng/ul and pBAD-RBS-pSB1A2 is 50 ng/ul.
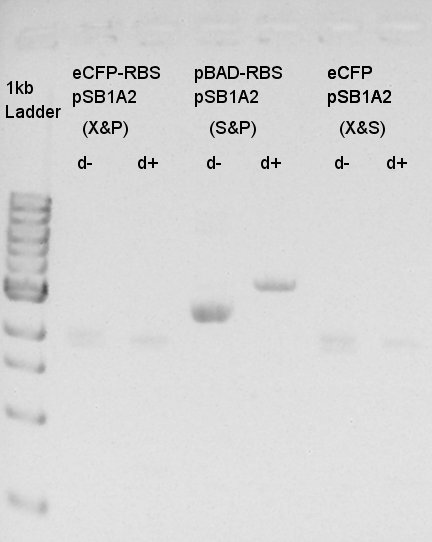
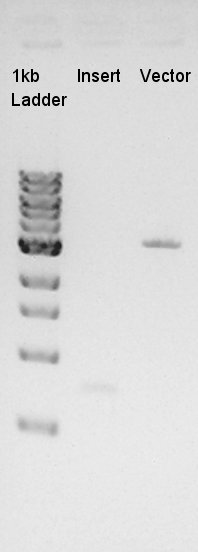
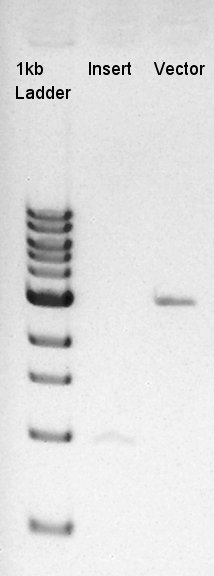
Digestion of eCFP-RBS-pSB1A2 and pBAD-RBS-pSB1A2
To make a construct of pBAD-RBS-eCFP-RBS-pSB1A2, we digested eCFP-RBS-pSB1A2 with XbaI & PstI and pBAD-RBS-pSB1A2 with SpeI and PstI. And we digested eCFP-pSB1A2 with XbaI and SpeI as a control for confirmation of the ability to digest.
Insert (eCFP-RBS-pSB1A2)
| DNA solution (30 ng/ul) | 22 ul |
| XbaI | 1 ul |
| PstI | 1 ul |
| 10xM buffer | 3 ul |
| DW | 3 ul |
| Total | 30 ul |
Vector(pBAD-RBS-pSB1A2)
| DNA solution (50 ng/ul) | 3 ul |
| SpeI | 1 ul |
| PstI | 1 ul |
| 10xH buffer | 2 ul |
| DW | 13 ul |
| Total | 20 ul |
control (eCFP-pSB1A2)
| DNA solution (30 ng/ul) | 5 ul |
| XbaI | 1 ul |
| SpeI | 1 ul |
| 10xM buffer | 2 ul |
| DW | 11 ul |
| Total | 20 ul |
| Number | Degree | Minute |
| 1 | 37 | 120 |
| 2 | 60 | 15 |
| 3 | 4 | HOLD |
From this image, we confirmed that DNA were digested into fragments and all of restriction enzyme worked.
September 4th
Plasmid extraction
Plasmid extraction of pBAD-RBS-Ag43-dT on pSB1C3. We used plasmid extraction kit of Nippon genetics: FastGene Plasmid mini kit and finally we eluted the DNA with 50 ul of elution buffer.
Ethanol precipitation of digestion products (eCFP-RBS and pBAD-RBS-pSB1A2) and estimation of concentration
- Added 5 ul of NaoAc, 1.5 ul of glycogen and 125 ul of 100% ethanol.
- Centrifuged at 15000 rpm, 15 min at 4C.
- Removed supernatant and added 220 ul of 70% ethanol.
- Centrifuged at 15000 rpm, 10 min at 4C.
- Removed supernatant and dried out at room temperature, after that added 10 ul of DW.
We estimated the concentration of ethanol presipitation products.The concentration of Insert DNA solution is about 20 ng/ul and Vector DNA solution is about 50 ng/ul.
Ligation of eCFP-RBS and pBAD-RBS-pSB1A2
| Vector DNA (50 ng/ul) | 1.5 ul |
| Insert DNA (20 ng/ul) | 4 ul |
| Ligation Mighty Mix | 6 ul |
| DW | 0.5 ul |
| Total | 12 ul |
Ligation reaction time was in detail below.
| Degree | Minute |
| 16 | 30 |
| 65 | 10 |
| 4 | Hold |
Transformation of pBAD-RBS-eCFP-RBS-pSB1A2
- Mixed 2 ul pBAD-RBS-eCFP-RBS-pSB1A2 ligation product to 50 ul of thawed competent cells on ice.
- Incubated on ice for 30 min.
- Mixed 350 ul of LB.
- Prepared and Labeled two plastic plates with LB plate medium which contained appropriate antibiotics (LBA).
- Plated 300 ul of the culture onto first dish and spread.
- Mixed 450 ul of LB to 50 ul of the culture and plated 300 ul of it onto second dish and spread.
- Incubated the plates at 37C for 25 hours.
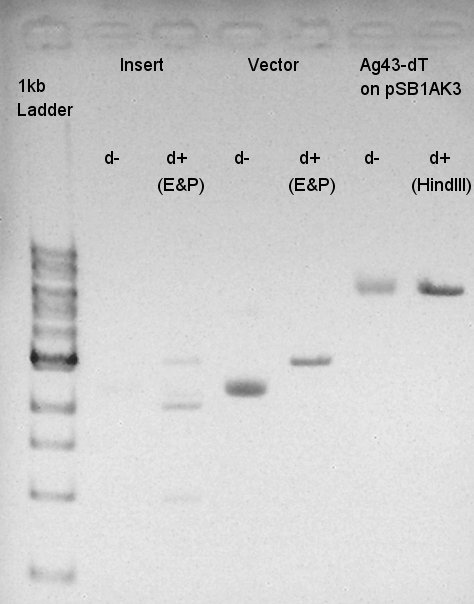
Digestion of ptet-RBS-eYFP-dT-pSB1A2 and pSTV28
To make a construct of ptet-RBS-eYFP-dT-pSTV28, we digested ptet-RBS-eYFP-dT-pSB1A2 with EcoRI and PstI, and pSTV28 with EcoRI and PstI. And we digested Ag43-dT-pSB1AK3 by HindIII.
Insert (ptet-RBS-eYFP-dT-pSB1A2)
| DNA solution (30 ng/ul) | 20 ul |
| EcoRI | 1 ul |
| PstI | 1 ul |
| 10xH buffer | 3 ul |
| DW | 5 ul |
| Total | 30 ul |
Vector(pSTV28)
| DNA solution (50 ng/ul) | 3 ul |
| EcoRI | 1 ul |
| PstI | 1 ul |
| 10xH buffer | 2 ul |
| DW | 13 ul |
| Total | 20 ul |
Ag43-dT-pSB1AK3
| DNA solution (30 ng/ul) | 30 ul |
| HindIII | 2 ul |
| 10xM buffer | 5 ul |
| DW | 13 ul |
| Total | 50 ul |
| Number | Degree | Minute |
| 1 | 37 | 120 |
| 2 | 60 | 15 |
| 3 | 4 | HOLD |
We confirmed that ptet-RBS-eYFP-dT-pSB1A2 was partially digested and pSTV28 would be successfully digested we think. But we couldn't confirm whether Ag43-dT-pSB1AK3 was digested or not. We did a gel-extraction of these digested DNA and got 50ul solution of each DNA.
September 5th
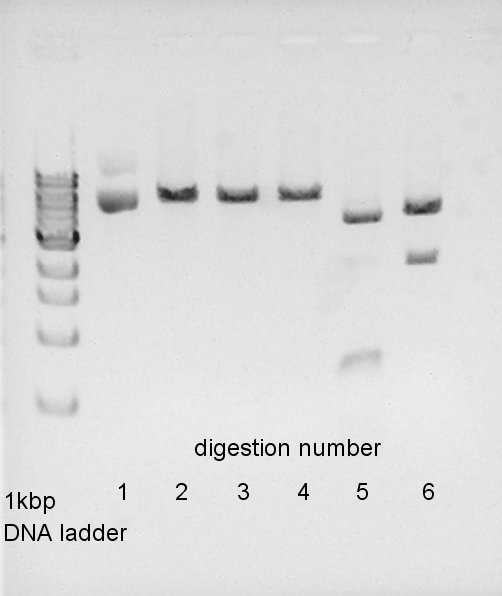
Digestion
We digested plasmid extraction products (pBAD-RBS-Ag43-dT on pSB1AK3) by restriction enzyme. Because I'd like to check the size of insert and vector of getting plasmid.
No. 1
| DNA solution | 1 ul |
| EcoRI | 1 ul |
| 10xH buffer | 1 ul |
| DW | 7 ul |
| Total | 10 ul |
No. 2
| DNA solution | 1 ul |
| XbaI | 1 ul |
| 10xM buffer | 1 ul |
| 10xBSA buffer | 1 ul |
| DW | 6 ul |
| Total | 10 ul |
No. 3
| DNA solution | 1 ul |
| SpeI | 1 ul |
| 10xM buffer | 1 ul |
| DW | 7 ul |
| Total | 10 ul |
No. 4
| DNA solution | 1 ul |
| PstI | 1 ul |
| 10xH buffer | 1 ul |
| DW | 7 ul |
| Total | 10 ul |
No. 5
| DNA solution | 2 ul |
| EcoRI | 1 ul |
| SpeI | 1 ul |
| 10xH buffer | 2 ul |
| DW | 14 ul |
| Total | 20 ul |
| Number | Degree | Minute |
| 1 | 37 | 120 |
| 2 | 60 | 15 |
| 3 | 4 | HOLD |
September 6th
Ag43 expressing
We usually used plastic tube for incubating.
The E. coli expressing Ag43 attachment to the face of tube wall.
We estimated the reason is attachment to hydrophobic materials. We incubated in 1 ml of LBA (one contained 1% of L-arabinose, another did not contain L-arabinose) at 37C in glass tubes.
Ethanol precipitation of ptet-RBS-eYFP-dT-pSB1A2 and pSTV28
- Added 5 ul of NaoAc, 1.5 ul of glycogen and 125 ul of 100% ethanol.
- Centrifuged at 14000 rpm, 30 min at 4C.
- Removed supernatant and added 220 ul of 70% ethanol.
- Centrifuged at 15000 rpm, 15 min at 4C.
- Removed supernatant and dried out at room temperature, after that added 10 ul of DW.
We confirmed that the concentration of Insert DNA solution is 10~20 ng/ul and Vector DNA solution is 30~40 ng/ul.
Ligation of ptet-RBS-eYFP-dT-pSB1A2 and pSTV28
| Vector DNA (30~40 ng/ul) | 1.5 ul |
| Insert DNA (10~20 ng/ul) | 4 ul |
| Ligation Mighty Mix | 6 ul |
| DW | 0.5 ul |
| Total | 12 ul |
Ligation reaction time was in detail below.
| Degree | Minute |
| 16 | 30 |
| 65 | 10 |
| 4 | Hold |
Transformation of ptet-RBS-eYFP-dT-pSTV28
- Mixed 2 ul ptet-RBS-eYFP-dT-pSTV28 ligation product to 50 ul of thawed competent cells on ice.
- Incubated on ice for 30 min.
- Mixed 350 ul of LB.
- Incubated for 2 hrs to get the resistance to Chloramphenicol.
- Prepared and Labeled two plastic plates with LB plate medium which contained appropriate antibiotics (LBC).
- Plated 300 ul of the culture onto first dish and spread.
- Mixed 450 ul of LB to 50 ul of the culture and plated 300 ul of it onto second dish and spread.
- Incubated the plates at 37C for 19 hrs.
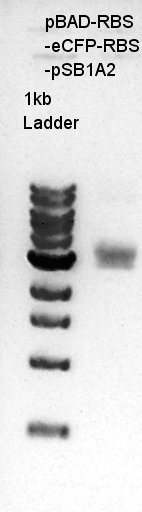
Colony PCR of pBAD-RBS-eCFP-RBS-pSB1A2
| DNA solution | 4 ul |
| Kapa-Taq(Taq polymerase) | 5 ul |
| Forward Primer(pbad-f2 primer) | 0.5 ul |
| Reverse Primer(PS-R down primer) | 0.5 ul |
| Total | 10 ul |
| Number | Degree | Second |
| 1 | 95 | 120 |
| 2 | 95 | 30 |
| 3 | 53.3 | 30 |
| 4 | 72 | 60 |
| 5 | 72 | 60 |
| 6 | 4 | HOLD |
Cycle:2~4 x 35
We used N1 (DW only) as controls. Desired product is about 900bp.
We thought that almost all of ligated DNA successfully ligated to our desired construct. We selected No. 1 colony for incubation and store No. 2,3 and 4 colony liquid medium at 4C.
Incubation of pBAD-RBS-eCFP-RBS-pSB1A2 for plasmid extraction
- Prepared 2 ml LBA into culture tubes.
- Re-suspended 1 colony mixture.
- Incubated at 37C for 18 hrs.
Transformation of ptet-RBS-eYFP-dT-pSTV28 and Test to confirm appropriate antibiotic amount for screening of pSTV28
To confirm how amount of chloramphenicol is needed for screening pSTV28 (Low copy plasmid) , we plated transformed cells on the special LBC which contains half amount of chloramphenicol we usually add. Now we call this LBC plate medium as LB1/2C.
- Mixed 2 ul ptet-RBS-eYFP-dT-pSTV28 ligation product to 50 ul of thawed competent cells on ice.
- Incubated on ice for 30 min.
- Mixed 350 ul of LB.
- Incubated for 2 hrs to get the resistance to Chloramphenicol.
- Prepared and Labeled two plastic plates with LB plate medium which contained appropriate antibiotics (LB1/2C).
- Plated 300 ul of the culture onto first dish and spread.
- Mixed 450 ul of LB to 50 ul of the culture and plated 300 ul of it onto second dish and spread.
- Incubated the plates at 37C for 19 hrs.
September 7th
Estimation of concentration of pBAD-RBS-eCFP-RBS-pSB1A2
We estimated the concentration of pBAD-RBS-eCFP-RBS-pSB1A2 is about 30 ng/ul.
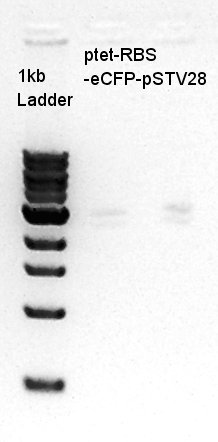
Incubation of ptet-RBS-eYFP-dT-pSTV28 for plasmid extraction
- Prepared 5~6 ml LBC into culture tubes.
- Re-suspended 2 colony.
- Incubated at 37C for 7 hrs.
 "
"