Team:Freiburg/Project/Overview
From 2012.igem.org
Pablinitus (Talk | contribs) (→Review of existing TALE construction methods) |
Pablinitus (Talk | contribs) (→Review of existing TALE construction methods) |
||
| Line 25: | Line 25: | ||
3. The third category of TALE assembly protocols applies Golden Gate Cloning (GGC)<sup>5,6,7,8,9</sup> (for details on GGC, see the Golden Gate standard page). In all GGC-based TALE repeat assembly strategies, level 1 modules (i.e. single repeat gene fragments) are flanked by type IIs restriction sites adjacent to their first or last 4 nucleotides, respectively, that produce sticky ends after digestion with the type IIs restriction enzyme. Since each level 1 module codes for the same amino acid sequence (despite of the RVDs), the codon usage must be changed at these 4 external nucleotides for producing unique sticky ends that assemble in the predefined order after digestion. Consequently, the 4 bp overlaps of a level 1 module specify its future position within the TALE gene. | 3. The third category of TALE assembly protocols applies Golden Gate Cloning (GGC)<sup>5,6,7,8,9</sup> (for details on GGC, see the Golden Gate standard page). In all GGC-based TALE repeat assembly strategies, level 1 modules (i.e. single repeat gene fragments) are flanked by type IIs restriction sites adjacent to their first or last 4 nucleotides, respectively, that produce sticky ends after digestion with the type IIs restriction enzyme. Since each level 1 module codes for the same amino acid sequence (despite of the RVDs), the codon usage must be changed at these 4 external nucleotides for producing unique sticky ends that assemble in the predefined order after digestion. Consequently, the 4 bp overlaps of a level 1 module specify its future position within the TALE gene. | ||
So, in order to be able to target any sequence of DNA, a method that is using GGC requires N x M modules. N signifies the number of level 1 module positions (i.e. number of modules that the TALE should contain after GGC) and M signifies the number of different repeats that the user should be able to put into each of the N positions (in most kits M equals 4, one repeat for each DNA base). | So, in order to be able to target any sequence of DNA, a method that is using GGC requires N x M modules. N signifies the number of level 1 module positions (i.e. number of modules that the TALE should contain after GGC) and M signifies the number of different repeats that the user should be able to put into each of the N positions (in most kits M equals 4, one repeat for each DNA base). | ||
| - | Unfortunately, using GGC, only up to 10 modules <sup>5</sup> can be assembled with high accuracy. So in the GGC-based protocols, level 1 modules get assembled to form level 2 modules (oligorepeats). These level 2 modules need to be amplified and isolated before a second GGC reaction assembles them to form the complete repeat array. The bottleneck of the GGC-based methods is the need for amplification and isolation of level 2 modules, which costs a lot of time, requires some extra knowledge, additional enzymes and lab equipment (we actually tried one of the GGC-based open source kits, but, even after 2.5 weeks, were not able to assemble the whole TALE). | + | Unfortunately, using GGC, only up to 10 modules <sup>5</sup> can be assembled with high accuracy. So in the GGC-based protocols, level 1 modules get assembled to form level 2 modules (oligorepeats). These level 2 modules need to be amplified and isolated before a second GGC reaction assembles them to form the complete repeat array. The bottleneck of the GGC-based methods is the need for amplification and isolation of level 2 modules, which costs a lot of time, requires some extra knowledge, additional enzymes and lab equipment (we actually tried one of the GGC-based open source kits, but, even after 2.5 weeks, were not able to assemble the whole TALE).<br><br> |
== GATE Assembly == | == GATE Assembly == | ||
Revision as of 22:49, 26 September 2012
The GATE Assembly Kit
Therefore we herein describe the Golden Gate cloning-based TAL Effector (GATE) Assembly platform, which enables literally everyone to produce low-cost, tailored TALEs within a few minutes of labwork and basic lab equipment. Moreover, we have automated this strategy and produced different TAL Effector Transcription Factors with 96 % success rate faster than any other method published before.
Review of existing TALE construction methods
Although TALE assembly is considerably easier than e.g. screening for novel zinc fingers, the highly repetitive structure of the TALE gene implies some challenges, because conventional PCR or homologous recombination-based gene assembly strategies cannot be applied. To our knowledge, the numerous approaches TAL-Effector gene assembly, published so far, fall under the following Three categories:
1. Few groups have applied methods called unit assembly1 or Restriction Enzyme And Ligation (REAL)2. In the first step both strategies perform conventional restriction enzyme digestion in order to assemble gene fragments of single repeats. The pairs of repeat gene fragments are subsequently assembled to form tetramers, and this highly hierarchical assembly strategy is continued until the desired number of repeats is assembled. These platforms obviously involve multiple laborious and time consuming rounds of digestion, ligation and isolation of the right ligation products. The recently published fast ligation-based automatable solid-phase high-throughput (FLASH) system circumvents major challenges of REAL by attaching the first repeat to streptavidin-coated magnetic beads and, successively, adding further repeats or oligorepeats from a 376-plasmid library. Although Reyon et al. claim that FLASH can also be performed manually, this probably does not represent the most convenient and low-cost protocol for iGEM students.
2. We call the second category of TALE production methods the synthesis optimization approach. The major challenge of TAL synthesis is the highly repetitive amino acid sequence of the DNA binding part. Since synthetic genes are typically produced from overlapping synthesized oligos, overlaps of different pairs of overlapping oligos need to be distinct. The synthesis optimization approach employs a sophisticated computer program that optimizes codon usage in order to reduce repetitiveness of the TAL gene and calculates optimal oligos for synthesis3,4. Although this approach might be the method of the future, it is currently too expensive for iGEM teams.
3. The third category of TALE assembly protocols applies Golden Gate Cloning (GGC)5,6,7,8,9 (for details on GGC, see the Golden Gate standard page). In all GGC-based TALE repeat assembly strategies, level 1 modules (i.e. single repeat gene fragments) are flanked by type IIs restriction sites adjacent to their first or last 4 nucleotides, respectively, that produce sticky ends after digestion with the type IIs restriction enzyme. Since each level 1 module codes for the same amino acid sequence (despite of the RVDs), the codon usage must be changed at these 4 external nucleotides for producing unique sticky ends that assemble in the predefined order after digestion. Consequently, the 4 bp overlaps of a level 1 module specify its future position within the TALE gene.
So, in order to be able to target any sequence of DNA, a method that is using GGC requires N x M modules. N signifies the number of level 1 module positions (i.e. number of modules that the TALE should contain after GGC) and M signifies the number of different repeats that the user should be able to put into each of the N positions (in most kits M equals 4, one repeat for each DNA base).
Unfortunately, using GGC, only up to 10 modules 5 can be assembled with high accuracy. So in the GGC-based protocols, level 1 modules get assembled to form level 2 modules (oligorepeats). These level 2 modules need to be amplified and isolated before a second GGC reaction assembles them to form the complete repeat array. The bottleneck of the GGC-based methods is the need for amplification and isolation of level 2 modules, which costs a lot of time, requires some extra knowledge, additional enzymes and lab equipment (we actually tried one of the GGC-based open source kits, but, even after 2.5 weeks, were not able to assemble the whole TALE).
GATE Assembly
|
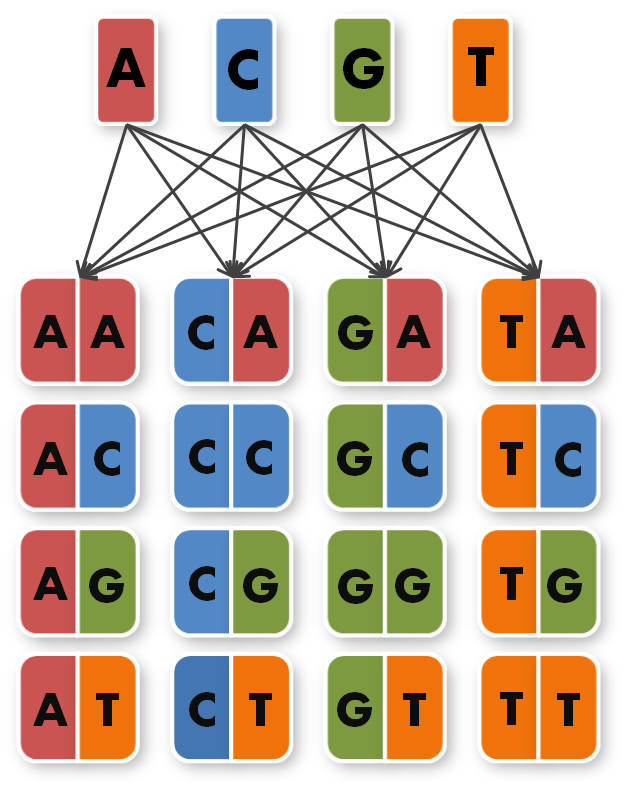
We took the sequences of the four already known TAL repeats A,C,G,T and combined them into 16 new, so called direpeat sequences. These 16 direpeats were ordered as gene synthesis products.
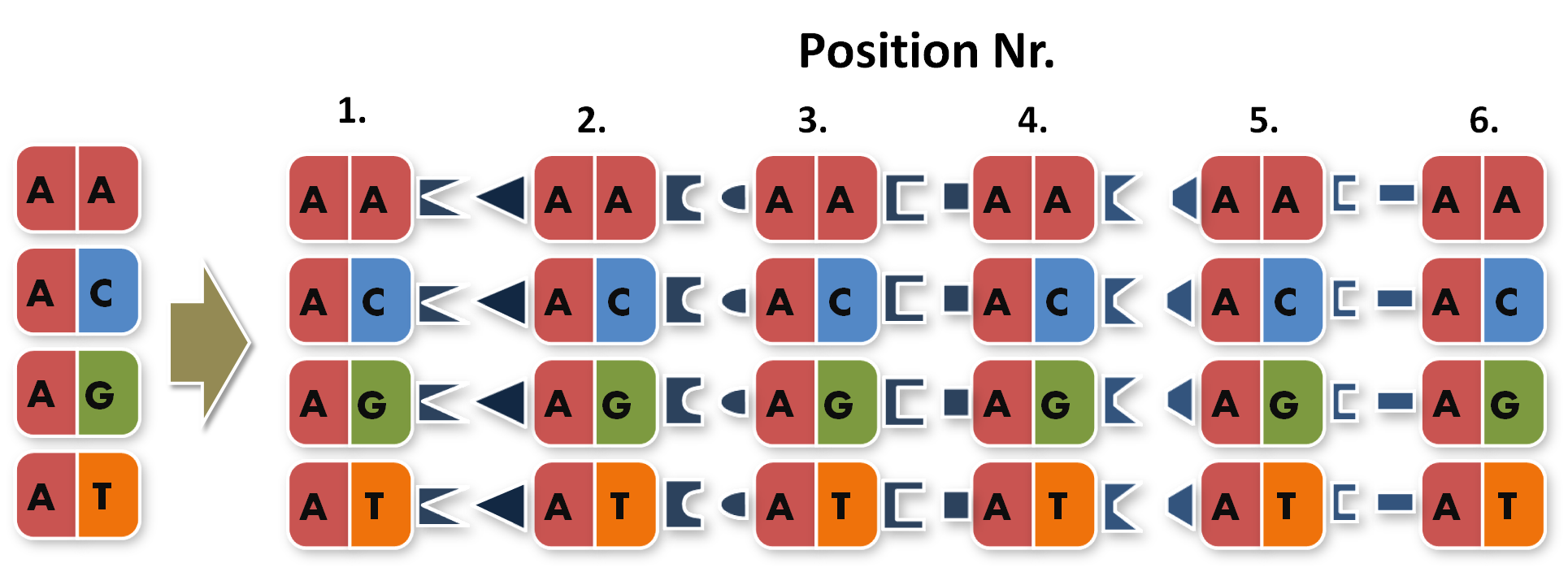
Now the real work began. To start building TAL Proteins we needed to expand our 16 direpeats a second time. Each of the 16 direpeats needed to be integrated into six versions marked with different terminal sequences, one version for every place of our final six direpeat TAL protein. Because we didn't want to buy six times 16 different synthesised direpeats we came up with a plan to produce them by ourselves. We created six primer pairs, each primer with a common part matching all of the direpeats and an unique overhang contacting the direpeat it binds to.
|
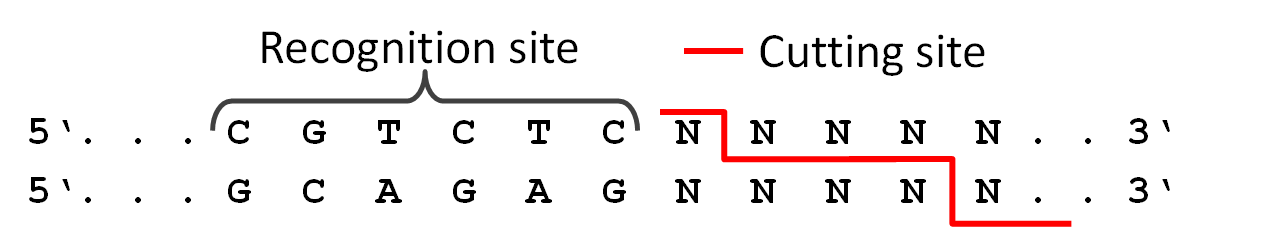
Because we did not feel comfortable requiring six different restriction enzymes in the final PCR to produce a twelve direpeat TAL, we used a technic called 'Golden-Gate Cloning'. The technic uses the type two restriction enzyme BsmB1 and its ability to cut DNA slightly downstream of the recognition site. This way it was possible for us to create different sticky ends with just one enzyme. Therefore we are able to ligate six different parts in the right order in one PCR step.
|
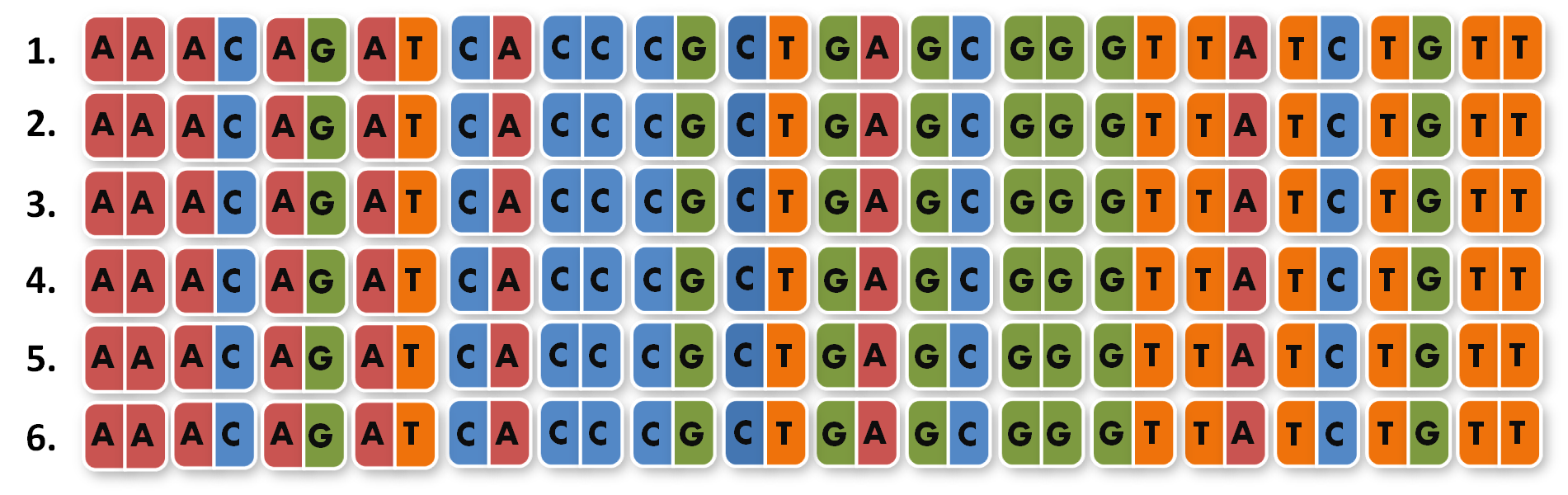
After finshing these 96 different extension PCR's we had to ligate the products into the orignial iGEM BioBrick vector and finally got our full library of 96 unique direpeats.
|
 "
"
